Health Strategy.
The strategy for the fight against cancer depends on which of the theories of cancer will be chosen as the working one. If the theory on which the treatment is based is incorrect, then most likely the treatment itself will not be successful.
Currently, there are several theories of carcinogenesis * * *. The dominant theory, despite growing criticism, is still the Somatic Mutation Theory (SMT) *.
According to SMT theory,
- the normal state of human cells is rest, not expansion;
- the appearance of a tumor is an accident;
- a tumor is a formed by abnormal (mutated) cells;
- a tumor arises from a single cell that has one or more mutations in genes that control cell growth and maturation;
- mutations of these cells are irreversible;
- mutations result in uncontrolled cell growth and other cancerous characteristics.
Based on these positions, the treatment of cancer should be the destruction of cancer cells by any possible means, from which the scalpel, poison and radiation are traditionally used. And the success of treatment depends on how completely the cancer cells are destroyed.
However, in order to selectively affect cancer cells without significant damage to normal cells, it is necessary to know the differences between cancer cells and normal cells.
It is customary to distinguish the following hallmarks of cancer:
- inflammation and fibrosis associated with the tumor;
- reprogramming of the energy risk of cancer cells, which forms an acidified hypoxic intercellular environment;
- the predominance of cellular signals over the growth of signals that inhibit growth;
- predominance of cell survival signals over death signals;
- stimulating the steady growth of new blood vessels (angiogenesis);
- genomic instability of cancer cells, causing high adaptability and vitality;
- functional inferiority caused by cell immaturity;
- the possibility of uncontrolled cell division;
- management of the cellular microenvironment, including immune cells;
- germination of cancer cells in neighboring tissues (invasion) and their spread to distant parts of the body with limited formation of tumors there (metastasis) *.
Cells that include all of these features are more commonly found as «cancer cells».
Uncontrolled division. The life and behavior of a cell of a multicellular being differs from the life and behavior of a single-celled being. The transition from a unicellular existence to a multicellular existence is a transition from hostility and competition of the same type of cells to mutually beneficial cooperation and cooperation in the complex organism. It is a transition from the complex and dangerous life of a single cell to a simpler and safer life at the cost of partially losing one's freedom and accepting the burden of obligations to other cells. It is a transition from individualism to collectivism.
In a multicellular organism, each individual cell follows not its own selfish interests, but the interests of the entire community of cells. Each mature cell performs its specific function, and obeys not only its own internal and environmental signals, but also the signals of neighboring and distant cells. Each individual cell communicates with other cells, sending them its messages, and receiving response information from them, which inclines them to certain actions, ranging from the «to divide» program to the «to die» program.
The signaling environment of the cell is filled with many conflicting instructions, but which one will be executed and which one will be ignored depends on their strength and balance.
A cell begins to divide when the signals that inhibit its division are inferior in strength to the signals that stimulate its division. Under normal conditions, when there is no need for the growth of organs or the organism as a whole, or for the rapid restoration of lost tissue cells, the signals that inhibit division are amplified. And in the case of, for example, trauma and the acute inflammation that accompanies it, there is a temporary increase in division signals. As a result, local tissue growth (proliferation) and reparation occurs.
In fact, cancer cells are cells that have lost their social function. Cells that have returned to a single-celled way of life with its immortality, anarchy and selfishness.
Apoptosis evasion. If the existence of a cell does not meet the public interest due to its inferiority or uselessness, it receives, respectively, internal and/or external commands to terminate its life. If the survival commands turn out to be weaker, then the process of apoptosis is triggered – the programmed «suicide» of the cell. However, cancer cells manage to develop mechanisms that allow them to either ignore commands to commit suicide or reinforce commands to stay alive.
High genetic plasticity. Stressful conditions for the existence of a cell enhance genomic instability, and genomic instability increases genetic flexibility, i.e. promotes multiple mutations in DNA, including those that allow the cell to adapt to survive in an unfavorable environment.
«Immortality». The stability of the terminal sections of DNA molecules is provided by special proteins, so-called telomeres. The number of telomeres that fix the stability of DNA is exhausted with each division of a mature cell, so it can divide a limited number of times. Complete depletion of telomeres leads to disruption of the normal process of division, and as a result, to cell apoptosis. Only stem cells have the ability to divide indefinitely due to their ability to regenerate telomeres.
Cancer cells, like stem cells, can stabilize the DNA molecule by increasing the activity of an enzyme called telomerase, which repairs telomeres, and thereby divide indefinitely.
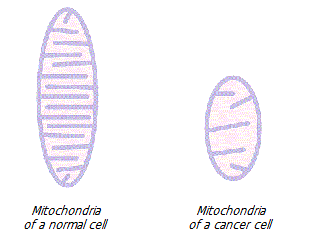
Metabolism modification. Cellular metabolism is the totality of all chemical reactions occurring in cells. Normal cells get almost all the energy they need through the oxidation of pyruvate, previously obtained from glucose glycolysis (aerobic respiration). Cancer cells most often obtain a significant portion of their energy by producing lactic acid from pyruvate, which does not require the presence of oxygen (anaerobic respiration). They do this whether or not there is enough oxygen for aerobic respiration, or not.
Inflammation associated with the tumor. Despite the variety of carcinogenic factors, most of them come down to chronic low-intensity inflammation that provokes and stimulates cancer. Inflammation, and the local tissue thickening associated with it, is now recognized as the central site of the entire oncogenic process. Cellular processes associated with inflammatory signaling play a major role in all stages of carcinogenesis, including emergence (initiation), development (progression), invasion (invasion), acquisition of new blood vessels (angiogenesis), and long-range spread (metastasis) of a tumor *.
Tissue invasion and metastasis. Benign tumors have a clear border, and grow, moving apart the surrounding tissues, but without damaging them. Unlike benign tumors, malignant tumors do not only grow in size. Cancer cells can eat away at the protein film surrounding the tumor, consisting mainly of collagen, and invade neighboring tissues. For this reason, they do not have a clearly defined border.
Cancer cells separated from the tumor can be carried by the internal fluid flow (lymph or blood) to distant parts of the body, forming new tumors there. And although almost all of them die in this difficult path, some of them still manage to survive, creating the risk of new tumor foci.
Formation of a quasi-organ. Any cell other than a normal host cell that comes into the view of normally functioning immune cells will sooner or later be detected and destroyed. This routine natural process occurs countless times in different parts of the body.
To avoid death, cancer cells have many different tricks – disguise; mimicry; shelter in a protected tissue niche; disorientation of immune cells and creation of unfavorable conditions for them; the formation of microspheres from similar cells, which increases their resistance. And finally – the formation of such an environment of normal cells, which will not work for the body, but provide cancer cells with protection, raw materials and energy.
In a certain sense, a tumor is not just a collection of rebellious cells. It is a complex living organism made up of different types of cells that work together to forage, defend themselves, and evade hostile environments that try to destroy them. And of course, the tumor pursues goals that are absolutely incompatible with the goals of the host organism. In this regard, it is akin to a bandit community that puts its existence and its corporate success above the public one, while parasitizing on society itself.
The moment when the body loses control over the processes in the microenvironment of a group of abnormal cells can be considered the moment of the onset of a cancerous tumor. However, a whole chain of long and interconnected events leads to it.
The most clearly observed structural, metabolic, functional and other differences between normal and cancerous cell/tissue are summarized in the table above. These differences allow a targeted attack on cancer cells while sparing normal cells.
Conclusions. Despite the presence of these distinguishing features, none of them is inherent exclusively in malignant cells, and not inherent in certain circumstances in non-malignant cells *.
- Enhanced cell growth is also observed in normal tissue during mass death of body cells due to some local catastrophes. At the same time, the rate of division of cancer cells is not higher than that of replacement cells in case of physical tissue injury or normal rapidly dividing cells, for example, in the intestine or bone marrow. The problem is not in increased doubling of cells, but in the fact that the construction/restoration of tissue occurs unnaturally.
- The ability to evade signals that induce apoptosis, as well as the ability to protect DNA through telomerase, is not higher in cancer cells than in normal stem cells. However, «immortality» is not an exclusive property of cancer cells; normal stem cells are also «immortal».
- Increasing energy production from glycolysis is also not a unique phenomenon in a cancer cell. The percentage of use of glycolysis by cancer cells is not higher than by the cells of an early embryo. In addition, some normal cells can either constantly use glycolysis for energy production (eg, neurons) or temporarily switch to this mode (eg, muscle cells, some immune cells, etc.).
- Genetic modifications are also natural and accumulate with age. The only difference between normal and cancer cells is that the latter are genetically more unstable, as a number of internal and external stress conditions contribute to this.
- Incomplete differentiation (functional immaturity) is also not a unique property of cancer cells. Normal stem cells are also not differentiated. After stem cell division, daughter cells undergo a stage of incomplete differentiation at different rates. Epithelial cells differentiate the fastest; Moreover, the cells of the mammary gland are completely and finally differentiated only during the last trimester of a woman's pregnancy. The problem with cancer cells is that they remain «immature adults», giving birth to similar «children».
- Invasion of adjacent tissues is not characteristic of normal cells that form various organs. However, it is observed in some non-malignant phenomena, for example, in the benign growth of the uterus beyond its natural location. In addition, an event similar to «invasion» can be called the penetration of immune cells into the site of infection in a particular tissue, which is a routine natural process. In all three cases, the same signaling pathways are activated.
- Distribution to remote places is also not a regular phenomenon for cells. But theoretically, it is possible if a normal undifferentiated cell enters the circulating body fluids. Under normal conditions, this will lead to the death of the «lost» cell.
From all this we can conclude that the characteristic of cancer is not the presence of any one of these specific features of the cell, but the fact that several of them are simultaneously manifested at once. A cancer cell is not a fundamentally special cell. In this regard, it does not differ from normal cell, just as a criminal does not differ from a law-abiding citizen. The difference between them is not so much in anatomy as in behavior.
The conclusion to which further reasoning will lead us is the following. A cancer cell is a cell that is forced to exhibit a behavior that is usually not characteristic of a normal cell in order to survive in extreme conditions. It is forced behavior that becomes character. This is ignoring the social purpose of the cell in the body.
In other words, cancer is a mechanism for the survival of cells in a hostile external environment, which they themselves, in the main, create. In this regard, the external manifestation of cancer can be considered not only as a problem of initial or acquired physical defect, but as a problem of cell disorganization, which generates a new cellular phenotype *. Like an unnatural course of normal processes.
However, the features of tumor cells listed above are still a set of puzzles, and not a complete picture. We need to know not the statics, but the dynamics of the process, the cause-and-effect relationships of its elements. Because only understanding how normal cells acquire the behavior of cancer cells can answer the question of how this process can be prevented, slowed down and, if possible, reversed.
Cancer is not a gunshot wound, it does not appear suddenly. The process of cancer development is multi-stage, and has a multifactorial causality. It is preceded by a series of gradual diverse changes in the tissues of a particular organ, which can last several years, and which do not always end in a malignant tumor. These pre-cancerous changes are triggered by a variety of factors, which are called carcinogenic *, and which will be discussed below †.
In a complex chain of interrelated intra- and extra-cellular risk factors, each of them can be both the initial cause and the effect of other causes. For example, a lack of oxygen in the tissue can be both a primary factor (due to poor blood supply) and a secondary one (due to its acidification due to glycolysis metabolites), and the loop of these interrelated processes can eventually switch to this pathology.
Other positive feedback loops can be more confusing. Short disturbances in the usual course of cellular processes cause the inclusion of compensatory mechanisms that allow you to bypass the resulting problems. However, they require additional metabolic reactions, which means additional energy consumption.
When problems disappear, cellular metabolism returns to normal. But in cases when they become chronic, the cell has to adapt to new conditions and rebuild its phenotype so as to more successfully respond to stressful challenges and survive in increasingly worse external and internal conditions.
Tumor initiation. Let's try to suggest one of the possible, schematically simplified descriptions of the development of the cancer process. In fact, they are much deeper, more complex, and can have countless other variations and emphases. The scenario described below only serves to show the complex interweaving of the course of cancer development.
Chronical stress. The cells, tissues, and organs of the body are constantly exposed to countless acute traumatic events. However, a living organism sooner or later restores the resulting damage.
Injury causes a number of local responses that promote cell division and replacement of dead cells with new ones. Inflammation forms a special, fenced-off zone in the focus of injury, in which conditions are created that are unfavorable for the life of foreign microorganisms. As the wound heals and the status quo is restored in the tissue, these temporary responses fade. Chronic injury by disturbing factors creates a picture of a non-healing wound, which creates the conditions for incessant cell division and accumulation.
A temporary increase in the number of cells in a particular organ can also be observed under normal, «transitional» conditions. For example, the growth of breast cells sensitive to sex hormones during the period of maturation of the female body. In these cases, a short-term increase in hormone levels that stimulates cell division of the ducts or lobules of the mammary gland is genetically regulated, and is accompanied by an appropriate level of hormones-companions and hormones-antagonists. Therefore, the processes of formation and maturation of the breasts and reproductive organs occur naturally and harmoniously in women, without causing negative consequences.
Another example is the control of the birth and death of uterine cells during the ovulation cycle by fluctuations in the levels of two antagonistic hormones, estradiol and progesterone, respectively. Maintaining a balance between them ensures a normal monthly cycle and the health of the reproductive organs. In some cases, when the level of estradiol, its carcinogenic metabolites or other estrogen-like manifestations is chronically increased, they are detected in some way, are not controlled by balances, are captured by only one side of the cell – their division.
An example of non-hormonal chronic injury is the increased acidity of breast tissue. Low-level acidosis is one of the most common conditions that causes major human degenerative diseases, including breast cancer.
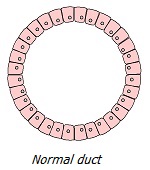
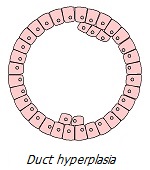
Chronic perturbation/irritation/injury leads to a number of phenotypic transformations of cells subjected to chronic stress. Outwardly, it looks like a consistent development of local abnormal conditions: hyperplasia (excessive division of normal cells) → metaplasia (replacement of cells of one type of tissue by another) → dysplasia (uncontrolled growth and loss of cell differentiation) → neoplasia (complete loss of tissue control over a growing neoplasm). Let us consider in more detail the chain of these transformations, which are clear symptoms of tumor processes invisible to the eye.
Proliferation. In normal tissues of an adult organism, a balance is maintained between the number of dying cells and the number of new cells that appear in their place. In other words, a balance is maintained between apoptosis (death) and proliferation (growth) of cells. But in the tumor process, this balance is chronically disturbed in the direction of proliferation.
Due to the heterogeneity of the structure of the organ/tissue and the uneven supply of cells, as well as due to the heterogeneity of the distribution of perturbing factors in it (intraductal papilloma, local thrombosis, calcifications, alcohol, viruses, bacteria, trauma, hormones), a critical coincidence of some of them at certain points the mammary gland has a much stronger local stress effect *. Such points can later become the center of tumor transformations.
Stress/injury disrupts mitochondrial function, which induces the expression of cell growth and tissue repair genes (GR genes). As a result, there is an increase in the production of growth factors and repair in order to heal the injury. In addition, these genes do more than just stimulate cell production. but also suppress apoptosis *. Thus, the balance of apoptosis:proliferation is shifted towards proliferation.
It appears that prolonged inability to endure a high stress load forces sensitive cells to increase their numbers in order to distribute the stressor to more cells. Similar phenomena occur with systematic intense physical activity, which stimulates an increase in the number of muscle cells and muscle mass. Thus, at some points of tissue sensitive to stimuli, there will be a significant increase in the number of normal cells (hyperplasia), which is a natural tissue reaction in response to external stimuli.
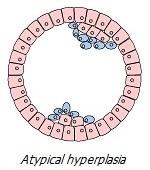
Breast hyperplasia is very common, and by the age of 70 the number of women with hyperplasia reaches 69% *. The widespread prevalence of hyperplasia, however, does not mean that this is normal. In the same way, the prevalence of atherosclerosis, hypertension, obesity, osteoporosis and diabetes does not indicate their normality.
If the perturbation of the tissue does not weaken, and the possibilities of proliferation are limited or exhausted, the response may be the replacement in the tissue of cell types that are more sensitive to a given stimulus by less sensitive types of normal cells of the same organ (metaplasia). In this case, cells typical for this type of tissue are replaced by atypical cells. A striking example of this is the replacement of ciliated epithelial cells in the lungs of heavy smokers with squamous cells. Similarly, there is a replacement of estradiol-sensitive cells of the epithelium of the ducts and lobules of the mammary gland by flat cells, which occurs at a more advanced stage of tumor formation.
Microscopic foci of hyperplasia, strictly speaking, are not dangerous, and when the conditions provoking them are eliminated, they may disappear over time. However, they temporarily change the structure of the tissue at the site of their appearance, increasing the risk of developing a tumor. Under certain conditions, hyperplasia can trigger a series of cross and parallel events that promote the tumor process *. Proliferating cells may lose the properties of normal specialized cells, and form atypical hyperplasia. Which over time, depending on the circumstances, can either normalize or become a precursor of a tumor – benign or malignant *.
Proliferation, eventually giving rise to a tumor, is thus a natural response to a traumatic factor. It is stimulated by the same signals that occur during injury and inflammation, and is aimed at repairing damaged tissue. The problem here is the chronic nature of the proliferation in an attempt to heal a non-existent, non-healing wound.
Excessive cell proliferation creates unfavorable conditions for the existence of cells. Nutrient concentrations decrease, hypoxia and acidity increase, which increases the risk of DNA damage and reduces its ability to repair *. However, uncontrolled proliferation is not enough for tissue to become cancerous; a simple example of non-malignant proliferation caused by a carcinogenic agent, papillomavirus, is a wart. For a malignant tumor, a violation of many other functions and processes, a qualitative degeneration of cells is required.
Thus, the carcinogenic effect of chronic stress on tissue can be represented as follows. In response to traumatic signals, cells trigger a series of natural reactions associated with injury healing. These reactions include the activation of growth and repair genes (oncogenes), the release of cytokines, the production of various growth factors, the division and differentiation of local and attracted stem cells, and the restructuring of tissues to ensure their vital functions.
In both trauma and tumors, we are dealing with similar processes of cell division in order to repair tissue. Only in the first case is it really necessary, but in the second it is a false necessity. In other words, in the first case, a temporary and controllable semblance of cancer leads to healing of the trauma, while in the second, permanent and uncontrollable trauma leads to real cancer.
In the case of acute injury, the process of restoration of damaged tissue is ultimately successful. At the site of injury/healing, undifferentiated cells undergo terminal differentiation * or apoptosis * and all non-functional cells and molecules are eliminated. In the case of chronic traumatic signaling, efforts to restore tissue do not stop. If signals of success do not arrive for a long time, then the cells understand this in such a way that the phenotypic capabilities they have are not enough to solve the problem.
The exhaustion of the possibility of overcoming existing challenges in simpler, quantitative ways inclines cells to solve them through qualitative changes. These changes are accompanied by genomic and metabolic modifications within the cells themselves, as well as reorganization of their surrounding tissue and reprogramming of the behavior of surrounding cells *.
Increased glycolysis. The stress load caused by pathogens directly suppresses the work of mitochondria – cell organelles. In addition, it increases local inflammatory potential, and inflammation decreases oxygen levels and increases free radical levels.
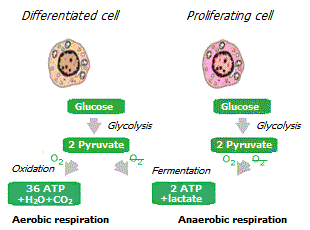
Oxygen is used by cell mitochondria to produce energy from pyruvate (pyruvic acid) by an electrochemical process called oxidative phosphorylation (aerobic respiration). With oxygen deficiency, cells cannot get enough energy from mitochondria. In addition, mitochondria can be degraded due to various reasons. Therefore, in addition to oxidative phosphorylation, cells have to use an alternative mechanism for energy production – fermentation of pyruvate outside the mitochondria, which does not require oxygen (anaerobic respiration).
The anaerobic method of energy production is several times less efficient than the aerobic one, therefore, to obtain a comparable amount of energy, it requires a multiple increase in the supply of glucose to cells and an increase in intracellular glycolysis. On the other hand, anaerobic respiration requires only one chemical reaction to extract energy from pyruvate, while aerobic respiration requires several (Krebs cycle). Thus, although glycolytic cells produce several times fewer ATP molecules from a single glucose molecule, they can produce ATP almost a hundred times faster and more easily just by increasing glucose consumption *.
The chronic increase in glycolysis and the shift in energy production from mitochondrial to enzymatic has dramatic consequences. Mitochondrial energy production is required for cell differentiation, so its fall causes loss of cell differentiation and unrestricted proliferation *. And enzymatic energy production is required for cell division, so its growth also promotes proliferation.
Several enzymes involved in glycolysis, such as hexokinase, are important regulators of apoptosis and gene transcription *. Activation of hexokinase causes it to move from the cytoplasm to the mitochondrial membranes, where it interacts with and suppresses several key components of mitochondria-dependent apoptosis *. Thus, glycolytic (including cancerous) cells can gain an advantage over non-glycolytic ones.
Increased glycolysis increases the concentration of pyruvate inside the cell. In a healthy cell, this pyruvate enters the mitochondria for energy production, metabolizing to water and carbon dioxide. But if the mitochondria cannot do this, for example, due to a lack of oxygen, then excess pyruvate accumulates inside the cell. This increases the activity of an enzyme called lactate dehydrogenase, which metabolizes pyruvate to lactate (lactic acid), which is then excreted from the cell into the extracellular space.
Acidification of the extracellular environment. The metabolites of aerobic respiration are water and carbon dioxide, which, when combined, form carbonic acid, which has a weak acid index (pH 3.68). And the metabolite of anaerobic respiration is lactic acid, which has an order of magnitude stronger acid index (pH 2.44). In addition, for the same level of energy production, lactic acid is produced many times more by anaerobic respiration than carbonic acid by aerobic respiration. And in order to maintain adequate intracellular acidity, it must be effectively removed from the cell. The removal of lactic acid from the cell is usually not difficult, which cannot be said about its removal from the intercellular space. With the accumulation of lactic acid, acidity in the tissues increases.
An increase in the acidity level in a solution leads to a drop in the level of oxygen in it, due to the fact that the alkaline environment contributes to the saturation of the solution with oxygen, while the acidic environment displaces oxygen. When cells increase enzymatic respiration, there is not only a lack of oxygen, but also an excess of toxic metabolites, including lactic acid. This increases intercellular acidity, which does not allow raising the level of oxygen in the cellular environment to a sufficient level. A chronic lack of oxygen does reduce the functionality of mitochondria and inclines cells to continue to make extensive use of glycolysis.
In addition, acidification of the extracellular environment inclines cells to become alkalized in the intracellular environment. And this, in turn, favors cell division. A decrease in intracellular acidity is seen during mitosis * because a more alkaline environment activates genes associated with protein production to create a new cell *.
Thus, increased oxygen-free respiration promotes acidification and destabilization of the tissue microenvironment, facilitating the path to tumor formation * *. Chronic oxygen deficiency may be enough for a normal cell to irreversibly switch its energy production mechanism and transform into a cancer cell. This was first demonstrated by Otto Warburg about a hundred years ago, which gave him reason to conclude: "The cause of cancer occurs whenever any cell is denied 60% of its oxygen requirements. Cancer, above all other diseases, may have countless secondary causes. But, the prime cause of cancer is the replacement of the respiration of oxygen in normal body cells by a fermentation of sugar" *.
Violation of the functions of enzymes. In every organism, and in every cell that forms its own system, a huge number of biochemical reactions, called metabolism, or metabolism, simultaneously proceed. The degree of intensity of each of these reactions requires individual management, because they are all interconnected in a complex way.
To ensure reasonable controllability of metabolism, living organisms use complex protein structures called enzymes. They speed up certain chemical reactions in the cell hundreds of times, and some of them make them fundamentally possible. Protein enzymes differ from other chemical catalysts in their extraordinary efficiency and extremely high specificity for a particular reaction. Such a solution makes it possible to supply energy and raw materials for the course of each specific process only to the reacting molecule *. Instead of supplying them to the entire system, as would happen in a test tube. Such a fundamental difference between the course of chemical reactions distinguishes living nature from non-living.
It is enzymes that determine which metabolic pathway biochemical reactions will take place. Violation of the delicate and balanced function of enzymes leads to biochemical anarchy and, as a result, to a wide variety of diseases.
Increasing glycolysis and flooding the tissue with the end products of its metabolism not only increase inflammation, but also change the composition and acidity of the chemical environment. Due to the fact that each enzyme is active in a rather narrow range of temperature and acidity, the work of the enzyme system is disorganized, which contributes to the release of biochemical processes in the tissue from control.
Violation of the functions of mitochondria. Increasing evidence every year suggests that cancer is more of a metabolic than a genetic disease. That in fact genomic mutations are not the root cause of cancer. They do not oblige the malignant degeneration of cells, but only contribute to it and accompany it.
Convincing evidence for this is provided by many laboratory experiments that contradict the prevailing theory of carcinogenesis. For example, there are non-genotoxic carcinogens that can cause cancer without DNA changes (such as chloroform and dichlorobenzene) *. A malignant tumor without any DNA mutations can also be provoked by introducing non-mutagenic foreign materials into the tissues of the body that disrupt cellular interaction *.
Replacing the nucleus of a normal germ cell with the nucleus of a cancer cell (with mutated genes) does not make it cancerous; normal organisms are subsequently born from such a cell *. Conversely, replacing the nucleus of a cancer cell with the nucleus of a healthy cell (without mutations) still leaves it cancerous. From the nuclei of cancer cells of brain tumors, healthy clones of mouse embryos were obtained *. From cancer cells introduced into early embryos, healthy tissues and organs are formed *. The organelles of the cell, not its nucleus, determines the malignant state of the cell * * *.
On the other hand, introducing functional mitochondria into a cancer cell can reverse its various malignant characteristics, including cell proliferation, hypoxic viability, anti-apoptotic properties, resistance to anti-cancer drugs, invasion and colony formation, and tumor growth *.
Many other similar experiments leave no basis for the claim that cancer begins with damage to the cell's nuclear DNA. It is not only the presence of mutations or the expression of oncogenes that makes a cell malignant. The state of health of its internal environment, including the functionality of mitochondria, plays a decisive role. Even the genetic mutations associated with malignancy cannot make a cell malignant as long as the mitochondria and other cell organelles function adequately.
Indeed, numerous data indicate that in some forms of cancer, chromosomal and gene mutations are absent * * *, and in other cases, changes in the nature of DNA methylation, but not in its sequence *, were found. At the same time, the appearance of malignant neoplasms and aerobic glycolysis follows the loss of mitochondrial function *. In addition, all cellular signs of cancer can be associated with dysfunction of mitochondria and energy metabolism *.
Chronic hypoxia caused by various reasons can lead to damage and destruction of proteins necessary for the normal functioning of mitochondria. Hypoxia increases the production of free radicals (oxidants), but this is not the only cause of oxidative stress. Many free radicals are formed during mitochondrial respiration, and there are many factors for its degradation. Although free radicals are important signaling molecules, their excessive concentration destroys any protein structures.
Free radicals not only damage mitochondrial DNA (mtDNA), increasing the risk of mutations; they also cause damage to the mitochondrial inner membrane and leakage of calcium from the mitochondria into the cytosol *. When severely damaged mitochondria are not adequately removed, their content leaks into the cytosol and then into the extracellular environment. Since mDNA does not correspond to nDNA, mitochondrial proteins are recognized as foreign, which generates and enhances the inflammatory process *. Chronic inflammation itself increases nuclear DNA genetic instability * and mitochondrial dysfunction *. As a result, both of these processes (free radical production and mitochondrial dysfunction) mutually feed off each other, creating a vicious circle.
Mitochondrial DNA is more sensitive to oxidative stress than nuclear DNA. Because of this, there is a high level of mtDNA mutations in cancer cells *. The mitochondrial genome encodes many important components of the respiratory chain proteins, so mutations in mtDNA can negatively affect the adequacy of cellular respiration by stimulating pyruvate fermentation *. The number of mtDNA copies is also reduced, and may fall below a critical level, which promotes the growth and metastasis of tumors *, including the breast tumors *.
Mychondria dysfunction can be caused not only by oxidative stress and hypoxia, and the resulting mitochondrial DNA mutations, but also by many other reasons. For example, the natural aging of mitochondria, chemicals, radiation, viruses, and other carcinogens.
Finally, last but most important. Mitochondrial dysfunction is largely caused by high blood glucose due to overnutrition. To rid the blood of excess sugars, insulin forces cells to take up glucose even though they don't actually need it. Sugar is sticky in nature, and the part of the sugar that does not participate in glycolysis sticks to the proteins that are present in the cell. For example, to enzymes, which worsens all metabolic processes. The attachment of glucose to other proteins also causes disruption of their function. In addition, such (glycated) proteins may be considered a foreign agent by the immune system, increasing the level of systemic inflammation.
Cells try to get rid of excess glucose by metabolizing it into fatty acids, which are then stored directly inside the cell. However, intracellular accumulation of triglycerides is directly related to mitochondrial damage or dysfunction of oxidative phosphorylation *.
As a result of chronic stress, mitochondria undergo morphological changes, resulting in atrophy of the sites involved in oxidative phosphorylation. As the structure of an organ determines its function, ATP production falls. Due to the lack of energy, all processes that require ATP slow down. The synthesis of enzymes and the work of membrane transporters are weakened; cells lose their natural shape, organization and adequate intercellular communication.
The mitochondria send out chemical signals that they are not capable of providing enough energy, and alternative energy production pathways are needed. In response to them, the so-called oncogenes are activated in the nuclear DNA, which enhance glycolysis, thereby contributing to the malignant transformation of cells *.
In general, the functionality of mitochondria depends on the expression of many nuclear DNA genes that control energy metabolism, tumor suppressors, intracellular calcium levels, drug resistance, free radical production, and many other genes that can fundamentally change cell behavior * *. If mitochondria do not work well, and for this reason the cell requires less oxygen, and it continues to flow, then this oxygen, instead of being involved in mitochondrial energy production, is involved in the oxidation of cell proteins, increasing oxidative stress.
Mitochondria are also an important site for controlling autophagy and apoptosis *. Autophagy allows you to get rid of poorly functioning cells; they are simply eaten by immune cells. Apoptosis (the process of cell suicide) occurs, among other things, in the case of a defect in nuclear DNA. However, the deterioration of mitochondrial function can lead to the fact that abnormal cells with mutated DNA will remain alive. And generate the same abnormal offspring * with the same high levels of free radicals * that will cause new malignant mutations, and so on in a vicious circle.
Weakening of mitochondrial respiration and increased glycolysis reduces cell differentiation and stimulates cell division. Genes and signaling pathways involved in enhancing glycolysis are themselves anti-apoptotic * and will enhance the suppression of apoptosis in glycolytic cells. Interestingly, the total number of mitochondria in transformed cells can even increase, but their functionality still remains low.
The lack of energy leads to a decrease in the production of enzymes that repair and repair damaged DNA molecules, thereby accumulating mutations. The weakening of mitochondrial function causes telomerase to migrate from the mitochondria to the nucleus, where it strengthens nuclear DNA telomeres, which limit the number of cell divisions *. Due to this, the glycolytic cell, unlike a normal cell, gets the opportunity to divide an unlimited number of times. Or, as is often said, the cell acquires «immortality».
So, dysfunction of mitochondria leads to serious disorders of cellular health. It is an important cause of malignant cell transformation because it forces cells to turn to enzymatic energy production. Not all carcinogenic factors cause gene mutation, but almost all of them somehow impair the functioning of mitochondria. It seems that mitochondrial disruption is the common biological basis for the whole variety of cancerous tumors.
Drop in transmembrane potential. The lack of cellular energy impairs the work of ion pumps, which maintain the necessary concentration of certain substances inside the cell. Because of this, the ratio of extracellular and intracellular ions is disturbed. The number of negatively charged ions inside the cell drops, resulting in a decrease in the electrical potential across the cell membrane, called the transmembrane potential (Em).
Cells are not only a chemical, but also an electrical machine. The biochemistry of processes in a living cell cannot be considered in isolation from bioelectrical signals. Ion fluxes, voltage gradients (transmembrane or transepithelial), and electric fields are important regulators of cell behavior and tissue organization, controlling cell number (proliferation and apoptosis), cell position (migration and orientation), and cell identity (differentiation trajectory) *.
The transmembrane potential is considered to be a key parameter mediating the control of proliferation, the state of differentiation and malignant transformation of cells * *. At rest, mature, differentiated cells have higher Em values compared to their same Em value in the dividing state, as well as compared to undifferentiated cells such as embryonic, stem, or cancer cells. The drop in Em (depolarization) triggers a number of changes associated with the transcription of genes that are important for the phenotypic transformation of a normal cell into a cancer one *.
From this point of view, cancer can be viewed as a dysregulation of the information field that controls the activity of individual cells in the direction of normal function *.
Loss of intercell communication. An increase in the concentration of metabolic products of glycolytic cells (lactic and succinic acids) due to an increase in tissue acidity supports chronic local inflammation.
Inflammation increases the amount of proteins and polysaccharides that make up the extracellular matrix (ECM) – such as collagen and hyaluronic acid. ECM serves as a microframework that allows cells to attach to it and form an elastic tissue volume, and not spread like jelly. However, excessive accumulation of the extracellular matrix hinders the transport of signaling molecules to neighboring and distant cells. Increased adhesion of epithelial cells to the matrix reduces their sensitivity to intercellular signals and allows bypassing many of the normal pathways of growth suppression *. This is good for acute inflammation, when cells urgently need to divide in order to restore dead cells. But it is bad for chronic inflammation, which can contribute to the development of a tumor.
Epithelial cells are covered with so-called glycocalyx. This is a fur-like thin layer of oligosaccharides, polysaccharides, glycolipids and glycoproteins introduced into the cell, as well as cell adhesion molecules – immunoglobulins, integrins, cadherins, selectins. The glycocalyx connects the cell to the extracellular matrix and affects the ability of cells to form chemical or electrical signaling channels among themselves. Thus, the glycocalyx itself affects how information is filtered and sent from one cell to another.
Excess glycoprotein mucus can be eroded by proteolytic enzymes or weak alkalis. In normal tissues with a slightly alkaline index, and even more so with sufficient levels of enzymes, the glycoprotein layer is thin, and provides direct cellular communication with the help of cell adhesion molecules and the so-called «gap junctions». The cell's default aspiration is division, and not rest *, as is commonly believed so far. However, a normal dividing cell of a multicellular organism, receiving signals from close contact with neighboring differentiated cells, understands that it has nowhere to grow further, and stops its further division.
In the foci of hyperplasia, a different picture may be observed. An aggressive extracellular environment forces cells to defend themselves against it, including by building up their own glycocalyx. This is facilitated by the increased acidity of the intercellular space, which impairs the work of proteolytic enzymes that can dissolve excess glycocalyx. The accumulation of glycoprotein and extracellular matrix proteins in the intercellular space impairs the direct transmission of chemical signals between cells through special channels connecting them. The close contact signals that held back cell division are weakened. Thus, cells receive an additional stimulus for their growth.
An increase in the number of immune and other attracted cells at the site of inflammation can also negatively affect the signaling interaction of breast epithelial cells.
Prolonged deterioration of intercellular signaling affects the genetic mechanisms of cells in the focus of hyperplasia *. Due to the genetic and epigenetic influence of its microenvironment, oncogenes are able to transform the cell phenotype into a malignant one, in which control of growth, differentiation, and apoptosis is lost.
It all looks like the transforming cell is returning to its pre-collective past. With the loss of interaction with other cells, the benefits of living in a cellular community are lost, while selfishness can provide the cell with more favorable conditions for survival. In fact, cells primitivize and return to their primitive phenotype, which existed in pre-oxygen, pre-multicellular evolutionary times.
Loss of differentiation. Differentiation implies the acquisition by an immature cell of certain structural and functional characteristics that enable it to perform its specialized task in a multicellular community *.
This process can be compared to the human stage of «growing up». Just like people, a newborn, immature cell acquires a phenotypic specialization for its service in a particular organ and tissue. Embryonic cells are initially primitive, undifferentiated, largely anaerobic and multiply rapidly without restriction, while fully differentiated, specialized cells in normal tissues are aerobic and their division and growth are severely limited *.
As the embryo grows, the embryonic cells undergo morphological changes, increasingly different from each other, to form certain organs of the new body. A very small part of them remain in a poorly differentiated state – these are the so-called stem cells of a particular organ. Being in secluded corners, in the so-called niches, protected by a dense network of extracellular matrix, the stem cell acts as a queen bee in the hive, producing new clones of itself as needed. There is room for only one cell in the niche, and after division, one of the daughter cells leaves it and migrates to the place where the order received from. Here it is fixed, acquires its functionality and proceeds to its duties.
The formation of specific cell skills occurs by changing the expression of its genes under the influence of general and local signals. In the figure below, we can see how specialized immune cells emerge from a single stem cell through sequential differentiation.
The compaction of the extracellular matrix makes it difficult for communication between cells *. The situation is exacerbated by the accumulation of toxins inside the cells. All this disrupts the mechanism of sequential expression of genes that ensure the full maturation of cells. In the foci of hyper- and metaplasia, the number of cells with reduced (to varying degrees) differentiation increases, and the degree of cellular differentiation can be used to assess the progression of cancer.
Due to the incomplete maturation of the epithelial cell, it does not perform its functional role well enough in the organ intended for it. This can stimulate cell division to try and compensate for the low efficiency of workers by increasing their numbers.
Inferiority in the development of cell structures impairs the work of all its other functions, including those providing local tissue control over its reproduction. Epithelial cells are very quickly renewed – within a few days, so defective cells can supply their quantity very quickly.
Further primitivization, ignoring command requirements and, in the end, the uncontrollability of individual cells leads to the fact that they begin to resemble unicellular parasites. In other words, cells that have lost differentiation (primitivized cells), despite having a multicellular genotype, acquire a unicellular phenotype. Such cells perform their social function either very poorly or not at all, but actively consume resources, poison the environment, and multiply intensively.
To a certain extent, the environment of normal cells is still able to provide primitive cells with a sufficient level of signals that keep them from brutally antisocial behavior. And forcing them to obey the commands of apoptosis, control of division and differentiation. However, the gradual increase in the size of the nodes of hyperplasia in the mammary gland, or the fusion of closely spaced nodes of hyperplasia, increasingly weakens these inhibitory signals.
Deaf to external commands, such cells can migrate to places where they are not needed. Or stay in place, forming local accumulations of their own kind (mammospheres). When estrogen levels in the tissue are high (or under other conditions that increase blood clotting), fibrin coats the mammospheres, making it more difficult for immune killer cells to recognize and destroy nonfunctional cells.
Chronic compaction and disorganization of the extracellular matrix gives rise to many tumor-forming events * *. Cells interacting with a more rigid extracellular matrix proliferate more in response to growth factors *. The binding of cells to the extracellular matrix can suppress the activity of many tumor suppressors that keep the cell in a state of cellular dormancy, removing restrictions on cell division from it *. In addition, enhanced adhesion to the extracellular matrix directly or indirectly impairs the function of certain tumor suppressors that oversee cell cycle checkpoints *. For this reason, the mechanism of apoptosis is disrupted when errors occur during cell division, and defective cells are more likely to produce defective offspring.
Gradually, the cycle of all the interrelated phenomena discussed above is accelerated and aggravated: inflammation; fibrosis; oxygen deficiency; an increase in the proportion of glycolysis in cellular energy and acidification of the cellular environment; disorganization of the work of enzymes; loss of mutual cellular regulation; uncontrolled division; insufficient differentiation of cells; decrease in mitochondrial functions, leading to genetic mutations and negative evolutionary selection. This process is not one-time, but moves in branched closed loops, strengthening and deepening over and over again, and at times, vice versa, weakening.
When compensatory mechanisms fail to cope with growing challenges, biological processes in the tissue begin to spiral out of control.
Chronic inflammation. Any wound, whether acute or chronic, triggers the natural regeneration response of the damaged tissue *. With mechanical tissue damage, the very first phase of repair is hemostasis. At the same time, blood vessels narrow, and platelets, in contact with damaged blood vessel cells, form blood clots (thrombi). Both the first and second prevent blood loss and form a temporary physical barrier between the injury site and intact tissue.
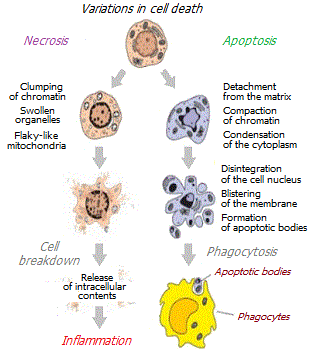
Injury or stress can be caused by causes other than gross physical trauma, such as infection. In this case, the death of body cells can occur without blood loss.
Almost immediately after hemostasis, the next phase of repair begins – inflammation. Damage to cells is accompanied by their mechanical destruction or death by necrosis. With necrosis, the contents of dead cells are poured into the intercellular space, causing inflammation. The inflammatory response is accompanied by local symptoms such as swelling, redness, warmth, and pain.
The blood vessels at the site of injury dilate and their endothelial cells become activated. The distance between them increases, and the rate of blood flow slows down. Due to this, signal molecules and immune cells seep through the endothelial layer into the tissues.
Immune cells of the first line of defense – leukocytes (white blood cells) absorb and remove foreign microorganisms, and natural killers – dead and damaged cells and their fragments *. Damaged epithelial and endothelial cells and fibroblasts release pro-inflammatory molecules (cytokines and chemokines) that summon and activate leukocytes (neutrophils, macrophages, and eosinophils), lymphocytes (T-cells, B-cells) and blood vessel cells to the site of inflammation *.
Chemical signals from injured tissue increase the expression of genes associated with cell growth. Mast cells, T cells, macrophages, and fibroblasts accumulating at the site of injury secrete growth and repair factors that promote cell division and repair of injured tissue. These are, for example, transforming growth factor (TGF-β), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and epidermal growth factor (EGF). It is noteworthy that the same genes and growth factors contribute to the emergence and promotion of cancer.
Blood clots resulting from hemorrhage impair fluid movement and oxygen delivery. This increases the concentration of hypoxia-induced growth factor (HIF-1α), which further stimulates cells to divide. At the same time, a temporary lack of oxygen does not impede the process of cell division, which use glycolytic energy production.
After a hastily made fence of the repair area and inflammation, the next phase of repair begins – proliferation.
Inside the wound layer, macrophages stimulate the synthesis of extracellular matrix (ECM) molecules by fibroblasts – collagen and glucosamine, which create a more reliable and lasting isolation of the damaged tissue repair site. As a result, the site of injury is compacted, and the pressure inside it increases.
Excessive accumulation of attracted cells and connective tissue (fibrosis) impairs the movement of special signaling proteins – chalones, which are produced by mature cells. Chalones block the section of DNA that triggers the process of division (mitosis), so their depletion further weakens the blocking of the process of tissue cell division. Hormones can also influence the rate of cell growth: estrogen stimulates the proliferation of breast tissue, while androgen inhibits it *.
Stimulated by growth factors, epithelial cells actively divide and restore damaged mucous membranes. Growing, they move along the damage surface from the edge to the center, and when they meet, they stop their division due to the action of contact signals. Activated fibroblasts (myofibroblasts) stimulate wound contraction; damaged tissue is replaced by connective tissue, forming scars. And endothelial cells form new blood and lymphatic vessels that provide oxygen supply and turnover of substances necessary for these processes. All together, this contributes to the rapid restoration of the tissue of the injured area.
If the source of injury is no longer there, then at the end of the proliferation phase, the expression of oncogenes (growth and repair genes) stops, and the expression of tumor suppressor genes begins. The last phase of repair begins – remodeling and maturation, during which inflammatory cells respond at the site of injury, the newly created cells finally differentiate, the processes of dissolution of the extracellular matrix begin to prevail over the processes of its synthesis. Excess collagen and blood vessels are absorbed, and scar tissue increases strength.
In previously damaged tissues, the normal organization of fibers, blood supply and lymph outflow are restored, tissue density is normalized. This repair phase occurs approximately 3 to 4 weeks after the acute injury and usually lasts up to 2 years. However, the tissue usually never recovers to its original appearance, and the remaining scars may not resolve for the rest of your life.
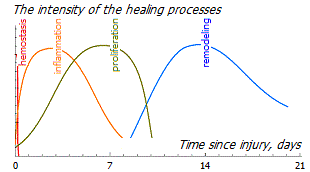
Healing is characterized by a strict sequence of precisely programmed biological processes. All four phases and their physiological functions must occur at a certain time and for a certain period with optimal intensity. If this process proceeds smoothly, as described above, then the period of inflammation and production of growth factors lasts a limited time, after which a short anti-inflammatory period occurs, and homeostasis is restored.
However, if healing does not occur properly, or the source of injury does not disappear, or tissue injury occurs more often than the process of its restoration is completed, then the sequence of physiological repair processes is lost *. If during the natural course of the process, at the end of regeneration, the «hang up» command sounds for the cells participating in it, and they stop their activity, then when the stages of the process are confused, signal chaos sets in.
In this case, the signals of the inflammatory and proliferative stages of healing can be superimposed on one another. The process of tissue repair cannot understand exactly when it should stop. It will tighten, creating an excess accumulation of cells. And if the stage of remodeling never occurs, the process can hang in this uncertainty for a long time.
If healing does not occur, then inflammation and fibrosis become permanent, and the production of growth factors continues, stimulating the constant division of cells prone to glycolysis, and creating conditions for the formation of a tumor. The mass of cells increases faster than their supply, and some of them die by necrosis, maintaining and increasing inflammation *. This closed cycle will be maintained either until the tissue is completely healed *, or until the organism is completely exhausted.
Similar, albeit less obvious, processes will occur with long-term exposure to any of the other carcinogenic factors discussed earlier that can create fibrosis.
Autophagy failure. Autophagy (translated from Greek – «self-eating») is a natural process of cleansing cells from internal reserves, from excess or unwanted elements inside cells; as well as the process of getting rid of weak, damaged or dysfunctional cells. This is a kind of cannibalism directed at oneself. Non-functional body proteins are disposed of and reused within the same organism.
Autophagy starts and works adequately only when the cell experiences a temporary lack of energy, and it has to turn to internal sources. Thus, long breaks in nutrition (daily fasts, 12-hour night abstinence, etc.) or calorie restriction (low-calorie diet, calorie mimetics, etc.) contribute to the internal cleansing of cells, as well as the removal of cancer cells.
Conversely, a high-calorie diet and a high level of circulating nutrients (glucose, amino acids, fatty acids) contribute to malignant cell transformation and the promotion of cancer. When the body receives enough food from the outside, then it does not need to resort to searching for internal possibilities.
On the other hand, excessive intensification of autophagy can lead to the fact that fully functional cells will be destroyed, and connective tissue will take their place.
A disorder of autophagy does not necessarily accompany the cancer process, but it can have a rather significant negative impact on it.
Formation of a precancerous niche. In chronic inflammation, a local change in the structure of the tissue occurs. As already mentioned, a chronic wound is characterized by a large amount of granulation tissue, which consists of a network of capillaries and an extracellular matrix, and which is enriched with fibroblasts, inflammatory cells, endothelial cells, pericytes and myofibroblasts. Attracted to the site of inflammation, inflammatory cells such as macrophages produce large amounts of growth factors, cytokines, and free radicals.
While free radicals are an effective way to kill acute infections, the continuous activation of macrophages poses a threat of tissue damage * and genetic mutations *. Some cytokines also develop transforming cells from apoptosis, which causes the production of the p53 gene *, which is called the guardian gene. In addition, inflammatory cells can help transforming cells evade the immune response * *.
The aggressive end product of anaerobic respiration, lactic acid (lactate), together with hypoxia-induced growth factor, stimulates the production of extracellular matrix. Chronic excessive production of collagen, proteoglycans and fibronectin on the one hand, and the weakening of the process of destruction of the extracellular matrix, on the other hand, leads to its accumulation. Excess extracellular matrix, in turn, promotes cell growth.
When the processes of cell division overtake the processes of their differentiation, there is a local accumulation of undifferentiated cells. Undifferentiated cells increase the production of growth factors, which could help injury heal, but in chronic injury/inflammation, this promotes tumor formation. On the other hand, signals to stop dividing come from mature cells more strongly, so the enrichment of the precancerous niche with immature cells enhances the carcinogenic tendency. Due to this, a self-sustaining cycle of uncontrolled reproduction of immature (transforming, tumor-forming, and then cancerous) cells arises.
An inexhaustible source of renewal of normal tissue cells are stem cells, which take refuge in a dense network of extracellular matrix, just as fish fry take refuge in the thick of algae. The extracellular matrix creates not only a reliable fence for the normal stem cell niche. It provides it with the flow of biochemical processes that contribute to protection, survival and further stay in an undifferentiated state.
Likewise, chronic, abnormally high fibrosis associated with inflammation increases the volume and density of the matrix, which forms the same safe haven harboring undifferentiated and mutated cells *.
Thus, thanks to these two processes – inflammation and the fibrosis caused by it, a specific local microenvironment is formed – a precancerous niche that inclines cells to increase glycolysis and division, and reduce their degree of differentiation.
Morphological disorder. As a result of the described processes, cells in the foci of tumor transformation gradually lose their normal appearance and structure, which can be seen under a microscope. The amount of connective tissue increases, structural anomalies of the entire tissue complex (dysplasia) begin to manifest themselves clearly.
However, mild dysplasia is still a reversible condition. It can not only progress, but stabilize, and even regress over time. Severe dysplasia already has few differences with the onset of a benign or malignant non-aggressive tumor.
Precancerous conditions are very common. A post-mortem study of the breast of middle-aged Danish women, conducted in 1987, showed a wide spread of diseases such as benign hyperplasia (55%), atypical hyperplasia (9%), metaplasia (85%), fibroadenoma (23%) *. Most of the lesions occurred in women over 40 years of age, and they apparently formed over many previous years. Most of the tumors did not exceed 5 mm in size, and most of these lesions were not detected until the death of these women. Over the past 33 years since the publication of this study, the situation with the prevalence of precancerous conditions, judging by the statistics of cancer incidence, has only worsened.
In the USA in the same years, 50% of women during their lifetime, and 90% of women posthumously, were found to have various fibrocystic conditions *. The problem has grown to the point that, due to the rapid surge in incidence, there have even been proposals not to consider fibrocystic conditions as a disease in the absence of atypia *. In other words, it was proposed to reduce the status of the disease due to the inability to cope with its epidemic.
In passing, we note that the aging of the body is accompanied by the same events – tissue oxidation, a decrease in mitochondrial functions, latent chronic general inflammation * and tissue fibrosis in many organs *. Therefore, any measures aimed at rejuvenating mitochondria, reducing the level of free radicals, counteracting fibrosis and reducing inflammatory signals in the body, have the potential to also slow down the aging process.
Genetic modifications can accompany the entire process of carcinogenesis, starting long before the appearance of the tumor itself. They are a consequence of the genomic instability of cells caused by their unfavorable external conditions, and the attempt of cells to adapt to these conditions through genetic and epigenetic changes.
Genetic modifications may play an important role in tumor formation and resistance, but mutations are actually only part of the problem, not the center of it. In many cases, it is not possible to detect somatic mutations in tumor cells, which clearly does not correspond to the generally accepted theory. However, in most cases, the malignant process goes so far that it leads to mutations, including in genes that contribute to the emergence, survival and reproduction of cancer cells (so-called oncogenes).
Despite their name, oncogenes and proto-oncogenes are expressed not only in cancer * and, as discussed earlier, in the initial stage of injury healing *, but also in pregnancy and embryonic development * *. The proteins expressed by these genes are temporarily involved in natural, non-cancerous processes.
The problem with oncogenes, therefore, is not that these genes that the body needs are expressed in principle. The problem is that they can manifest out of control – at the wrong time and in the wrong place. Adaptation to external conditions due to genetic adjustment with the further survival of the most successful options is the very mechanism due to which the evolution of living nature takes place. But in cases like cancer, this mechanism is detrimental.
Despite the fact that the development of a tumor is accompanied by an increasing number of genomic changes, it cannot be argued that they are the cause of cancer. Rather, they are the result of this process. Any diseased or abnormal cell in the body, including cancer cells, will be immediately recognized and destroyed by healthy immune cells. The development of a tumor is possible only with a certain combination of several unfavorable factors; the genetic factor alone is clearly not enough for this.
Subordination of the microenvironment. Overcoming the deterrent effect of the microenvironment of the precancerous niche and subordinating it to one's own interests is an important stage in the development of cancer. The influence of the microenvironment may have a stronger influence on the formation of the cell phenotype than the instructions of the genome *. Even multiple mutations of a single cell, with an adequate microenvironment, do not lead to the formation of a tumor. Expression of oncogenes generates a malignant phenotype only in a specific context of the microenvironment*.
Glycolytic cells immerse not only themselves in a toxic environment, but other normal cells surrounding them. Glycolysis energy production increases extracellular acidity, and high acidity leads to oxygen deficiency. Hypoxia and inflammation in the precancerous niche suppress the function of immune control, due to which defective cells elude detection and destruction. High local tissue density contributes to this, because under these conditions the function of immune T-cells and natural killer cells deteriorates.
While in the pretumor niche, signaling communication between cells decreases through direct contact, communication through exosomes, extracellular vesicles (EV), increases. Produced by tumor cells, exosomes carry certain types of immunoregulatory molecules into immune cells, allowing them to evade immune surveillance. Exosomes also form malignant phenotypes in other epithelial cells by carrying oncogenic proteins and miRNAs. Thus, tumor-forming cells, with the help of exosomes, modify the surrounding cells of the precancerous niche and create a favorable microenvironment that promotes tumor development *.
Part of the cells of the microenvironment can produce pyruvate from lactate produced by glycolytic cells, supplying them with scarce raw materials *. As a result of tumor-host interactions, in the microenvironment of the transforming breast tissue, and later in the tumor microenvironment, a kind of symbiosis of organ-forming cells of various degrees of differentiation and normal cells, including macrophages, fibroblasts, neutrophils, mast cells *… In this complex local ecosystem, its cellular and molecular elements influence the development and nature of cancer no less than the cancer cells themselves *.
Ultimately, the tumor microenvironment becomes a unique environment that controls all molecular and cellular events in the surrounding tissue involved in this environment. At this stage of cellular and tissue transformations, all previously hidden processes finally give rise to a tumor that we can somehow detect.
Weakening of immune control. The emerging tumor, under the guise of inflammation, also subjugates local immune cells *.
It is not easy for the immune system to recognize defective cells as its natural target. Firstly, for her, they are just not grown-up cells of her own body. Secondly, the identification of those antigens of mutated cells that are displayed on the cell surface is difficult due to the accumulation of a glycoprotein on it. In addition, exosomes secreted by hypoxic cells are able to change the expression of surface receptors that activate immune killer cells and prevent antigen recognition by dendritic cells. But this is only part of the problem.
The increased density of the extracellular matrix and increased pressure within the inflammation zone prevent the influx of oxygen and the outflow of lactate, the acidic waste product of rapidly dividing (glycolytic) cells. The dense extracellular matrix itself hinders movement and activity, and complicates the penetration of antitumor immune cells such as cytotoxic T cells and NK (natural killer) cells. In addition, extracellular matrix proteins call on immune-suppressing cells such as tumor-associated macrophages (TAM) * and regulatory T cells. The latter contribute to the survival of immature cells, believing that there is a process of tissue repair.
The increased acidity of the tumor microenvironment can weaken the immune surveillance of the tumor in several ways. For example, it weakens the activity of most enzymes, reduces tissue oxygen saturation and inclines cells to anaerobic energy production. In addition, antitumor cells such as T cells and NK cells tend to lose their function under high acid conditions *.
As a result of hypoxia, extracellular adenosine accumulates in the inflammatory zone, which suppresses the activity of T-cells *. In addition, a dense matrix impairs the outflow of the end product of anaerobic respiration – lactate, which is a strong disruptor of the normal functions of T-lymphocytes and natural killer cells – cells with a high glycolytic metabolism *.
Long-term high concentration of lactate switches the behavior of local macrophages from antitumor to protumor. Tumor-associated fibroblasts, seeking to reduce chronic inflammation, promote the acquisition of an immunosuppressive phenotype by local macrophages, neutrophils, dendritic and mast cells, as well as a decrease in the cytotoxic function of natural killer cells *.
They also promote recruitment and differentiation of myeloid suppressor cells *. In turn, myeloid suppressor cells curb the high activity of cytotoxic T cells and natural killer cells, promote angiogenesis, and also attract other immune-suppressing cells such as regulatory T cells *. Considering the focus of transformation as a place of injury and inflammation, they contribute to the restoration of damaged tissue through proliferation and angiogenesis, i.e. actually work for the development of the tumor, and not against it.
It can be said that due to inadequate understanding of the situation and their role in it, immune cells in the focus of transformation can take care of defective cells instead of eliminating them. All this makes it problematic to destroy mutated and defective cells by stimulating the immune system. Simply boosting immune cells will do little good. As a result, you can get only violent unproductive activity of a stupid performer.
The immune function is closely related to the material and energy supply of cells. The epithelial cells of the body are constantly and very quickly updated, which requires a sufficient amount of lipids and amino acids. When this building material is in short supply, processes such as autophagy are activated. At the same time, defective and poorly functioning body cells are eaten by macrophages and killer cells, and after their disassembly, assembly elements appear for building new cells. When the building material comes from food in excess, then immune cells are not stimulated to remove defective cells, including cancer cells.
Malignant transformation. So, inflammation and deposition of collagen and other extracellular matrix proteins are observed in the precancerous niche. The high density of collagen and the resulting high acidity and insufficient supply of oxygen and nutrients * favor the metabolic reprogramming of part of the breast cells in the direction of increased glucolysis and glutaminolysis *. An excess of extracellular matrix signals contributes to the loss of differentiation, i.e. primitivization of cells *; their self-renewal *; increased glycolysis *; apoptosis evasion *; genomic instability * and «immortality» *.
In other words, chronic inflammation followed by fibrosis promotes tissue reorganization in the precancerous niche and the acquisition by immature epithelial cells of all signs of malignant cells * *. Local malignant transformation of cells allows to increase the production of growth factors and tissue repair as the last desperate chance to heal a wound that never heals *. However, the result is completely different, since the wound does not heal, but on the contrary, thanks to the restoration efforts being made, it only increases.
Transforming cells divide, forming a cluster of cells with a specific local environment. This environment is characterized by: increased acidity; hypoxia; impaired cellular communication; high oxidative stress; high concentration of toxins, proliferative and inflammatory molecules… That is, such conditions under which the malignant phenotype is in more favorable conditions, and for this reason is finally fixed. In the future, even under favorable conditions (for example, with sufficient oxygen), the transformed cells and their descendants no longer voluntarily return to the previous phenotype (Warburg effect) *.
A positive feedback loop is formed: some cells inside some space protected and limited by the extracellular matrix, trying to adapt and survive in conditions hostile to them, continue to support and aggravate these hostile conditions. Moreover, they worsen the conditions not only for themselves, but also for other cells of this limited microenvironment, including immune cells. By highlighting various molecular signals, they also force the cells around them to adapt, change their phenotype and care about survival, and not about performing their functions. In this place, a non-functional accumulation of cells begins to appear, with the removal of which there are problems.
When the rate of division of the transformed cells outstrips the rate of their death, they accumulate, forming a kind of clusters (mammospheres) – the growth points of the future tumor. An environment of normal epithelial cells is able to inhibit malignant transformation of prone cells *. However, the appearance of an accumulation of transforming cells changes the microenvironment and removes this brake. The concentration of transformed (glycolytic) cells further increases local acidity, hypoxia, inflammation and fibrosis. Control of tissue processes in expanding mammospheres begins to either become distorted or lost.
In the end, what is called a tumor (neoplasia) arises - a neoplasm that has a tissue architecture and environment that does not ensure the normal supply and functioning/development of its constituent cells, creates serious problems for their survival, and poses a vital threat to everything body.
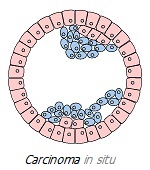
Early cancer (stage 0) is limited to the mucosa. For the mammary gland, an early tumor is carcinoma in situ. It is not yet malignant, but it is already a real tumor that can get a dangerous continuation. In most cases, such tumors are not detected until post-mortem autopsy or until growth, when they can be accurately diagnosed. A 1997 study found that ~9% of all women carry tumors in situ in their breasts, while only 1.3% of women of the same age are diagnosed with breast cancer *.
Invasion. Undifferentiated, for example, newborn cells, show detachedness and, as a result, a certain mobility. This allows it to migrate into tissues. For example, a normal epithelial cell moves to a dead cell replacement site, where it further differentiates and loses its ability to migrate. Differentiated cells are fixed in place by fouling with molecular bonds with neighboring cells and with the protein framework of the tissue – the extracellular matrix. The extracellular matrix is directly involved in both the healing of injury and tumor formation.
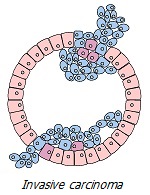
The protein layer tightly surrounding the tumor, consisting of collagens, proteoglycans and hyaluronic acid, is called the basement membrane. On the one hand, the basement membrane around the tumor creates a physical barrier that prevents the penetration of oxygen, many substances dissolved in the blood, including chemotherapy drugs, as well as circulating cells. On the other hand, this enclosure not only provides external protection for the tumor, but also restrains its spread.
Transformed cells that retain their differentiation to a certain extent will form a benign tumor. The cells of a benign tumor, while growing, are surrounded by a basement membrane, which limits their migration. Transformed cells that have significantly lost differentiation will form a malignant tumor, the cells of which are characterized by high invasion.
Tumor-associated macrophages (TAM) can secrete various proteolytic enzymes to destroy the basement membrane, thereby allowing tumor cells to escape from the environment and invade neighboring tissues and organs (invade).
The stronger the process of cell reproduction overtakes the process of their differentiation, the more primitive they are, the more glycolytic they are, the more invasive the tumor is, and the worse the prognosis of the disease. Although this aggressive behavior may take several years to manifest, it is indicative that the tumor has become malignant.
Self-sufficiency of proliferation. Cell doubling requires sufficient building material to build daughter cells. With a shortage of the necessary material, the division of normal cells can slow down. However, glycolytic cells are able to do without external delivery. Most of the necessary raw materials they receive from the metabolic products of the intermediate reactions of glycolysis in the cytosol of the cell *. In addition, some of the molecules it requires are provided by the mitochondria from by-products of glutaminolysis and oxidative phosphorylation.
Due to this, in a tumor environment, glycolytic (cancer) cells gain competitive advantages over normal cells for their reproduction. For their life and reproduction, it is enough for them to receive only glucose or glutamine, while for the life and reproduction of normal cells oxygen and other substances are required, the delivery of which to the tumor zone is difficult.
Tumor stamina. Among the variety of malignant cells, undifferentiated tumor initiating cells (TIC) are sometimes isolated. Since these cells exhibit many of the functional characteristics of normal stem cells, they have also been called cancer stem cells (CSC). CSCs are tumor cells with stem cell characteristics and initiate cancer formation.
The definition of «cancer stem cells» is based on three functional characteristics of these cells, namely:
1) the ability to initiate tumors when the containment potential of the tissue and the body is lost;
2) the ability to self-renewal, measured by the formation of tumors when they are transplanted into another organism;
3) the ability to differentiate into non-self-renewing cells that make up the volume of the tumor.
According to this theory, only a subset of CSCs are responsible for the onset, maintenance, and recurrence of a tumor, while the other cells of the affected organ, which constitute the bulk of the tumor, cannot self-renew or initiate tumor formation * *. And although CSCs make up a tiny fraction of the total number of malignant cells, they are an important factor in the persistence and progression of cancer * *.
Cancer stem cells are securely hidden in their niches; they are at rest most of the time; they do not need oxygen; they have an increased intensity of multidrug resistance membrane pumps; they have a low degree of differentiation. Thanks to their characteristics, CSCs show miracles of survival. They can renew themselves through epigenetic changes, acquire new mutations, and rapidly adapt to the environment, thereby forming a new tumor structure with molecular characteristics very different from those of the original tumor. They enable tumors to resist radiation and chemotherapy, create new clones of treatment-resistant cells, and ultimately cause recurrences in most types of cancer *.
The theory about the presence of CSC may be wrong, but it allows a simple and convenient way to explain the results of observations of processes occurring in the tumor.
Natural selection and evolution. With poor outflow of toxins (end products of glycolysis) caused by chronic fibrosis, glycolytic cells have an increasingly toxic environment around them. This forces them to further refine their phenotype, as only the most resilient and fittest of them survive and thrive. In other words, an acidic and hostile microenvironment favors the selection of cells that can survive in these extreme conditions, developing an increasingly aggressive tumor phenotype.
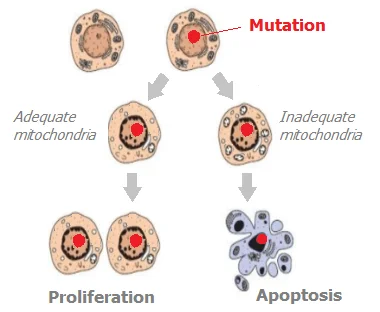
With low oxygen levels, the cellular mechanism for repairing DNA damage does not work well. Deterioration of environmental conditions also enhances the genomic instability of cells, and the instability of the genome produces mutations. In normal cells, genetic failures during cell division lead to the start of apoptosis by mitochondria, and the mutation dies along with the cell.
But in cancer cells, mitochondrial function is suppressed, and the mechanism for monitoring mitotic checkpoints is disrupted, which is why cancer cells are more resistant to internal signals of apoptosis. External signals of death by cancer cells, as already discussed above, are successfully blocked in the tumor zone, and the immune pathway for the elimination of mutated cells is weakened.
Taking advantage of such favorable conditions, mutations get a chance to continue in daughter cells. Most human cancers show two to eight consecutive genetic changes that develop over 10 to 30 years *. Each of these changes directly or indirectly increases the imbalance between the birth and death of cells in the direction of steady growth.
The mutation process is branching. Each mutated cell in the next generation not only inherits the parental mutation, but can acquire new original mutations *. Therefore, the number of different genetically modified populations (lateral subclones) multiplies in the tumor zone. The increase in the number of such populations of cancer cells increases the likelihood that some of the clones will be able to survive under the most unbearable conditions, including those created by radiation and chemotherapy.
Moreover, chemotherapy can increase the malignancy of the tumor. While the growth of normal cells in a toxic environment is inhibited, immature cells, which are more adapted to such adverse conditions, continue their division. Chemotherapy drugs that target fast-growing cells destroy most of the cancer cells that make up the tumor array. However, not all cells die, but only the weakest ones. Natural selection leaves the most successful cells, in fact, the most malignant of them, which are able to recur, better resist chemotherapy and significantly worsen the prognosis of the outcome of the disease.
However, genetic changes in nuclear DNA are not fatal. With normal functioning of intra- and inter-cellular signaling, mutations in nuclear DNA alone are not enough to cause cancer. It seems that with adequate cell functionality, oncogenic mutations do not have a chance to be realized, and that the structure of the tissue dominates the genome. If genes are not critically damaged, then a malignant tumor will not develop even in the presence of multiple chromosomal mutations *.
Increasing evidence every year suggests that cancer is more of a metabolic than a genetic disease. That in fact genomic mutations are not the root cause of cancer. They do not oblige the malignant degeneration of cells, but only contribute to it and accompany it.
Indeed, numerous data indicate that in several forms of cancer, chromosomal and gene mutations * * * are absent, and in other cases, changes in the nature of DNA methylation, but not in its sequence *, were found. At the same time, the appearance of malignant neoplasms and aerobic glycolysis follows the loss of mitochondrial function *. In addition, all cellular signs of cancer can be associated with dysfunction of mitochondria and energy metabolism *.
Even the transplantation of the nucleus of a malignant cell into a normally functioning cellular cytoplasm containing healthy mitochondria does not make the latter a malignant cell – normal tissues are subsequently formed from it * * *. Replacing the nucleus of a normal germ cell with the nucleus of a cancer cell (with mutated genes) does not make it cancerous; normal organisms are subsequently born from such a cell *. From the nuclei of cancer cells of brain tumors, healthy clones of mouse embryos were obtained *. From cancer cells introduced into early embryos, healthy tissues and organs are formed *. Conversely, replacing the nucleus of a cancer cell with the nucleus of a healthy cell (without mutations) leaves it cancerous. That is, the nuclei of normal cells in an unhealthy cell cytoplasm cannot cancel the malignancy of the cell.
On the other hand, introducing functional mitochondria into a cancer cell can reverse its various malignant characteristics, including cell proliferation, hypoxic viability, anti-apoptotic properties, resistance to anti-cancer drugs, invasion and colony formation, and tumor growth *. Thus, the malignant state of a cell does not depend on the nucleus of the cell, but on its organelles * * *.
Finally, cancer can be caused without DNA changes, for example, through exposure to non-genotoxic carcinogens (such as chloroform and dichlorobenzene) *. A malignant tumor without any DNA mutations can also be provoked by introducing non-mutagenic foreign materials into the tissues of the body that disrupt cellular interaction *.
All of this leads to the conclusion that, contrary to prevailing theory, it is not genetic mutations in nuclear or mitochondrial DNA that are the primary cause of a cell becoming cancerous *. It is not the presence of mutations or the expression of oncogenes that makes a cell malignant. Rather, it is the cytoplasm rather than the nucleus of the cell that determines the malignant state of the cell * *, and tumor formation requires the presence of ineffective mitochondria in the cells.
Thus, genetic mutations are not the center of the problem, but only part of the overall process. And the tumor phenotype can fundamentally be turned back to the normal phenotype * * *.
Angiogenesis. An increase in the production of lactic acid by glycolytic cells, and as a result, acidification of the intercellular space, reduces the concentration of oxygen in it.
In response to chronic oxygen deficiency and an excess of toxins, cells send signals demanding that blood and lymphatic vessels develop to them, and these vessels begin their formation (angiogenesis). In addition to hypoxia, there are other signals that promote angiogenesis. For example, estrogen, or chronic inflammation that accompanies the tumor process.
Angiogenic phenomena are typical of trauma, and they can appear in the tissue already at the very first stages of tumor formation *. The circulatory system begins laying new blood vessels, responding to persistent requests for 100-200 cells. Although the vessels grow into the tumor, and the local blood supply improves somewhat, the glucose supplied with the blood is again spent on glycolysis and an increase in the number of glycolytic cells.
Thus, the acidity of the tissue still remains elevated, and the problem of lack of oxygen is not solved, if not aggravated. The increased number of glycolytic cells demand even more vascular supply for themselves.
In addition, due to the perversion of signaling between cells, the newly formed vessels in the affected area are characterized by immaturity and incompleteness. A high percentage of fluid leaks from defective blood vessels and poor drainage of lymphatic vessels create a zone of local increased pressure. And it, in turn, impairs the supply of cells, and also prevents the flow of drugs and some molecules that provide remote intercellular communication.
Cells far from blood vessels receive the chemical supply they need, which is delivered by diffusion. This somewhat limits the high requirements of cancer cells for glucose. It is believed that with the diffuse method of nutrition, tumors cannot grow in size more than 1 mm, and then they are forced to stop their growth due to lack of resources. When supply pathways in the form of blood capillaries are laid to cancer cells, they get easy access to glucose, and thus can significantly increase their proliferative potential.
Many dietary components have anti-angiogenic properties †, so dietary modification to increase the proportion of plant-based and whole-grain foods may reduce tumor growth rates. In addition, adipose tissue is very saturated with blood vessels. Fat cells secrete large amounts of angiogenic factors and thus the growth of fat deposits increases the risk of cancer.
Metastasis is the process of cancer cells spreading beyond the primary tumor and forming secondary tumors in new locations, far beyond the primary tumor. Metastasis is the cause of about 90% of all cancer deaths *. At the same time, this process is still not entirely clear.
Differentiated cells are firmly attached to the matrix at their location. In order for adult cells to migrate, they will need to undergo some phenotypic transformation that will allow them to acquire motility. Such transformations are called the epithelial-mesenchymal transition (EMT). But the more primitive cells are, the less they are connected with the extracellular matrix, and the more they tend to be transferred with lymph or blood to near and far parts of the body, and form new colonies there (metastasis). Cancer cells just correspond to such characteristics, and the more aggressive the cancer cell, the more it is prone to expansion.
So, in normal tissues, the possibilities of cell migration are extremely limited. Cells from one organ do not voluntarily migrate to another organ, and if somehow this happens, they will be identified as foreign there and destroyed by immune cells. The same is true for circulating cancer cells. However, in this case, several difficulties arise.
Leaving the primary tumor, the transformed cells migrating to other organs carry a thick protein coating, which plays the role of masking and armor (e). The camouflage hides molecular marks on the outer surface of the cancer cell that would be recognized by immune cells as their legitimate prey. And the thickening of the membrane complicates the task of defeating and absorbing the metastasizing cell by immune cells – natural killers and phagocytes.
However, with a normally functioning immune system, a single metastatic cell outside of the tumor will be recognized (d), attacked and eventually eliminated. Breaking out of its microenvironment (a-b), it finds itself in adverse external conditions. Here, it is not covered by the jungle of extracellular matrix and inflammatory signals that suppress immune activity, and therefore has very little chance of survival. Successful metastasis requires the migration of not a single cancer cell, but a microscopic part of the entire tumor.
First, in this case, cancer cells, tightly accompanied by other cells of the tumor microenvironment, are better protected from aggressive external factors. Second, it is easier for them to avoid anoxia, a form of apoptosis that is caused by loss of attachment to other cells *. And thirdly, arriving in a new niche along with their microenvironment helps them to evade signals from neighboring cells of non-tumor tissue, which could incline them to restrain their reproduction, to apoptosis or to loss of malignancy (to the differentiation). A thick layer of sticky glycoprotein on the surface of cancer cells contributes to the formation of such a multicellular cluster, and lipoproteins additionally seal the gaps between them.
Tumor-associated macrophages (TAMs) can account for up to half of the total tumor volume. The tumor microenvironment is capable of changing the phenotype of macrophages in such a way that they lose the ability to absorb and assimilate immature (including cancerous) cells. Inactive, they continue to tightly surround the cancer cells, sheltering them from the still functional killer cells. Separating from the tumor array along with cancer cells, macrophages can provide the same protection for them during the journey.
If the penetration of cancer cells into the bloodstream (c) is accompanied by damage to the wall of the blood vessel, then the fibrin that appears at the site of injury will form platelet clots around the migrating cancer cluster, holding this micro-formation together. Additionally, a special protein, relin, which is found only in the brain, can be involved for cover. This allows the circulating cancer cell to cross the border between the blood and the brain, causing metastases in the latter.
Surviving after its dangerous journey, and stuck in a small blood capillary, migrating microclot with cancer cells loses some of its external protection. Exposure of its surface makes it easier for the cancer cell to fix to the local matrix, if it has not completely lost this ability. If the microclot in which it travels is large enough, it can impair local blood supply, and thus oxygen supply, creating a favorable environment for tumor development. If cancer cells arrive at a new site accompanied by immune cells that are predominantly glycolytic, they can further increase local acidity and hypoxia.
Since the penetration of an alien cell from a blood vessel into the tissue and the preservation of its malignant features requires a combination of certain conditions, foci of micrometastases do not necessarily immediately grow. They can stay in a frozen, inactive state for a long time, waiting for their finest hour. But most often they disappear due to unfavorable conditions for them and due to the work of functional immune cells.
Metastasis occurs most easily through the lymphatics. But distant routes of metastasis still pass through the blood vessels, despite more unfavorable external conditions.
At a site remote from the primary tumor, cancer cells need to travel back from the blood vessel to invade the local tissue (f-g). However, here the environment differs from the tumor. In a tumor, the blood vessels are poorly formed, corroded by aggressive cancer cell metabolites, and have a high permeability. Therefore, they easily allow the penetration of cancer cells into the blood. In healthy tissue, the walls of blood vessels do not favor the invasion of cancer cells if they are not previously prepared for this.
According to an alternative theory of carcinogenesis, the transport of cancer cells to distant places occurs with the help of macrophages. The absorption of hostile agents with their further decomposition and assimilation is the routine work of macrophages. However, when the functionality of macrophages is impaired in the inflamed tumor microenvironment, this process may not be carried out to the proper extent. For example, while maintaining the ability to absorb a pagegenic object (phagocytosis), macrophages may lose the ability to dissolve it (lysis).
For this reason, macrophages can dissolve the shell of an epithelial cancer cell, but retain its nucleus. As a result of this fusion, a multinuclear hybrid * is formed, which has the properties of both an epithelial cell and a macrophage *. With even greater dysfunction, being unable to assimilate the absorbed cell, the macrophage can preserve it entirely. Thus, macrophages can serve as vehicles for the transfer of cancer cells within themselves.
Something similar happens in some parasitic diseases, when parasites safely hide from their detection and death inside healthy cells of the body, for example, in erythrocytes. Thus, metastasis as a phenomenon can occur from the very first moments of the appearance of a tumor. And the reason for this may be the very immune cells that are designed to protect us.
Finally, according to the most radical version *, metastatic cells themselves originate from macrophages or other similar cells of myeloid origin (dendritic cells or lymphocytes). Malignant transformations occur in them due to respiratory failure and the aggressive microenvironment of the tumor in which they reside.
Macrophages are cells that do not bind to the matrix. They are genetically programmed to exist in the bloodstream and naturally penetrate tissue easily. Under their shell, cancer cells can escape from the tumor and be transported by the lymph flow to the lymph nodes. And the bloodstream is safe to spread far beyond the primary tumor, because the blood environment is natural for them.
The risk of metastasis increases with the growth of cell primitivization, and this process, apparently, begins already at the onset of a tumor. Even the smallest tumor is capable of regurgitating undifferentiated, sufficiently mobile tumor-initiating cells that can spread far beyond its limits.
As the size of the primary tumor grows, the number and size of migrating tumor-initiating debris increases. And although their chances of creating a colony of cancer cells in a remote place are minuscule (according to various estimates, only one out of tens or even hundreds of thousands of such attempts succeeds), because of the huge number of attempts, sooner or later, this happens.
At the time of diagnosis of the primary tumor, approximately 50% of patients already have undetected micrometastatic tumor colonies, which may eventually increase and lead to the return of the disease after its treatment. The larger the size of the detected primary tumor, the higher the probability of the presence of such colonies *.
If metastasis is the growth of cancer cells of the primary tumor in a new place, then any tumor, for example, in the lungs, that arose after a breast tumor, would be considered a metastasized breast tumor, and not a new lung tumor. But there are also alternative views. According to one of them, metastases arise due to the activation of the corresponding oncogenes in distant places by constantly circulating signaling molecules that originate from the primary tumor *.
In favor of this version, they give too short time for the development of metastases compared to the time for the development of the primary tumor. As well as the fact that plasma obtained from mice with tumors contains a high concentration of growth factors and heals wounds faster than plasma from healthy mice *. However, this point of view does not contradict the prevailing one. Perhaps, in a way that has not yet been clarified, the removal of the primary tumor by current treatment protocols can remove restrictions on the development of already spread metastases that are in a «sleeping» state.
Depending on the degree of invasion and metastasis, several stages of cancer are distinguished, which will be discussed further. Meanwhile, the primary tumor does not pose a mortal danger, unlike metastases, which are the main cause of death of cancer patients. This means that with timely diagnosis, we have time to take measures to slow down the tumor process and postpone the hour when metastases develop and kill the patient. Until death occurs from other causes.
The degree of danger. With the abundance of risks and negative factors that we can see, it seems surprising that cancer does not appear in all of us. Fortunately, the body is equipped with a large number of compensatory mechanisms to prevent this. Therefore, the drama with a tragic end described above is an extreme case, not a rule. Although the above processes occur in almost every adult woman, most often the body successfully overcomes the problems that arise. If this were not the case, the tumors would not have to mature for several years * * before they were discovered. The struggle can go with varying success, and its outcome largely depends on the behavior of the patient himself.
Malignant character traits do not absolutely take over the cells; cancer cells are still somewhat sensitive to external signals of apoptosis, growth inhibition, differentiation, and sedentary behavior. They can go into a state of relative dormancy and remain there for many years *. And even sufficiently large tumors can disappear without a trace on their own – such a rare phenomenon is called spontaneous remission. It can be assumed that at the micro level, transitions from benign to malignant conditions and vice versa can occur repeatedly, and imperceptibly both for the person himself and for diagnostics.
For the manifestation of tissue transformations that generate and maintain cancer, a temporary local disturbance of tissue/cellular balance is not enough. Proliferation may appear as calluses, warts, or papillomas, but is not itself the cause of the tumor. Local inflammation can continue for many years without causing tumors. Fibrosis can accompany the processes of inflammation and aging, causing a noticeable transformation of tissues, but not showing tumor signs. Genetic modifications are also not enough to cause cancer.
A simultaneous combination of several intra- and extra-cellular conditions is required, which in the long term are capable of forming self-sustaining carcinogenic loops. Eliminating even one of them at a very early stage can slow down the growth of malignancy. Elimination of a few – to transfer the movement to the rails of non-malignant conditions, and even reverse the process of the onset of a tumor. Which in fact is only the result of a violation of the normal functioning of cells and tissues.
The situation is different when the tumor has already formed. The multi-loop and multi-factor nature of the cancer process leaves no hope of finding a single drug formula, a single therapy, or a single goal that could break its entire mechanism. Breaking one or two causal links will be of little use, because due to their flexibility and adaptability, cancer cells will find mechanisms to bypass them. To defeat cancer, we will have to cut off all possible oncogenic chains in order not to give it a single chance. And even the creation of the most favorable conditions does not mean the voluntary return of cancer cells to a normal phenotype. And this means that they will need to be destroyed one way or another.
The formation and development of a tumor is not inevitable. Of the several thousand foci of hyperplasia, only a few form tumors. At the same time, most of them are not invasive, and accompany most women throughout their long and happy life. And even allow you not to think about asymptomatic painful processes occurring in the breast. But this is precisely what gives the cancer its guile and leaves him a chance to win.
"Cancer is a genetic disease. It comes from a single mutated cell and goes through the stages of origin, growth and metastasis." This is how medical textbooks are taught to this day. But as we can see from everything discussed above, this is not entirely true. Carcinogenesis is not a linear chain, but an intricate network of interrelated events. Mutations are involved in the process, and are only one of the fragments of the development of a malignant tumor. Cancer can be characterized not as an abnormal process in the body, but as an abnormal combination of natural cellular processes.
Returning to the fundamental positions of the theory of somatic mutations outlined at the very beginning of this section †, it can be noted that the picture of the development of cancer proposed here is in many respects a contraversion to it:
- the normal state of human cells is expansion (division and migration), and not rest;
- the appearance of a tumor is not an accident, but the result of a violation of the order of natural processes;
- a tumor does not appear from a single cell with one or more mutations, but from a group of closely spaced tissue cells that are exposed to traumatic signals that they are unable to reduce by conventional mechanisms;
- mutations of tumor cells are caused by the circumstances of the current process, and are not its initial cause;
- the negative consequences of mutations can be restrained by the normal intracellular state and the normal extracellular environment.
Now let's return to the position that the choice of a working theory of the origin of cancer directly follows the strategy of its treatment. If cancer is the result of hopeless errors in the genes, as the dogma still maintains, then eliminating all cancer cells is the only, and not encouraging, approach to treatment. This is the way of modern medicine.
If the change in gene expression is only a concomitant element of this process, and the cancer itself is the result of a dysfunction of intercellular interactions, then theoretically it can be reversed. The tumor can be induced to die, stop or significantly slow down its development, differentiate primitivized cells and form completely normal tissues by normalizing cellular signal
In particular, if we consider the tumor as the result of inadequate functioning, then such a vision of the problem involves a combination of the following local therapeutic manipulations to solve it:
- surgical removal of the main part of the tumor;
- decrease in the level of traumatic signals, including hormonal ones;
- decrease in the level of inflammatory signals;
- decreased resistance of cancer cells;
- restoration of mitochondrial respiration;
- stimulation of the final differentiation of cells;
- providing tissue with oxygen and building material necessary for the process of tissue repair;
- formation of healthy microflora in the epithelium;
- adequate adjustment of immune cells.
Concluding this chapter, it is impossible to get rid of the feeling of understatement. We can consider the mechanisms of the cancer process indefinitely, but we cannot say for sure what exactly is its actual cause and not a consequence. And are there any reasons at all?
There are many unanswered questions. Why, under seemingly identical conditions, do some people become its victims, while others do not? Why do some people develop cancer out of nowhere, while others don’t have it even with a lot of carcinogenic factors? Cancer still remains a mystery and, despite all the growing knowledge about it, something incomprehensible eludes our gaze. We see the process, but we do not see the driving force. Just as we don’t see the architect’s plan in his head behind the bustle of the builders. Just as we do not see the presence of the Holy Spirit in natural phenomena.