Health Strategy.
Hormonal balance is of utmost importance for women's health, including the health of the female breast. Hormones regulate vital biological functions of the body, including the cardiovascular, respiratory, digestive, reproductive, cerebral, and immune systems *.
Hormones are extremely powerful modulators of biochemical processes. Extremely low concentrations of this or that hormone are enough for it to produce its effect. However, they are only needed for a certain amount of time. The action of some hormones at the current moment can be compensated by the action of other hormones in the future. Thus, the level of hormones is constantly changing, depending on various circumstances. The main opposition of sex hormones occurs between estrogens and androgens, in particular, between estradiol and progesterone.
Each hormone plays its part, like a musician in an orchestra, and as a result of their common coordinated work, we get a symphony of a normally functioning organism. Hormones can control many physiological and even psychological phenomena. However, there are many reasons why the harmonious sound of hormones can be disturbed, resulting in a wide variety of diseases. Some subtypes of cancer are hormone-sensitive. There is still no consensus that estrogens are the cause of cancer, but they can be promoters of this process, and act in conjunction with both causes and other promoters.
Although elevated estrogen levels have been associated with an increased risk of breast cancer, the goal of hormonal modulation is not to unconditionally lower estrogen levels, but to balance the levels of antagonistic hormones. If a chronic imbalance in favor of estrogen is not confirmed by appropriate tests, then there is no need for hormonal modulation. But, unfortunately, this is not always the case.
Estrogen is the general name for a group of steroid hormones that in women are produced mainly in the ovaries, until the onset of menopause. Some estrogen is produced by the adrenal glands, and even less is produced in adipose tissue and the liver *. Estrogen can also be synthesized within the breast tissue itself from other steroid hormones *; this pathway becomes more active in postmenopause. In women, estrogen is a key factor in puberty, the menstrual cycle, and even behavior.
Estetrol (E4) is the weakest and thus the least dangerous estrogen. Its level becomes noticeably high only during pregnancy, because it is produced by the fetus. Pregnancy is believed to increase the risk of breast cancer in women in the short term. However, this risk may be more related to the increase in estradiol concentrations during pregnancy, while estetrol may play a protective role by pushing the more potent estrogens away from cellular receptors.
Estriol (E3) is approximately 80-90% of the total amount of female estrogen. It is biologically weak estrogen. The imbalance of estrogen, which results in a decrease in estriol, leads not only to obesity *, but also to the dominance of stronger estrogens.
Estrone (E1) is approximately 5-10% of total estrogen. It exhibits stronger binding to the estrogen receptor than estriol, although weaker than estradiol.
Estradiol (E2) also accounts for approximately 5-10% of total estrogen. It is the most powerful and dangerous estrogen because it is 12 times more potent than estrone and 80 times more potent than estriol. Excess fat mass in the body, as well as excess fat in the diet, contributes to an increase in the levels of estrone and estradiol in the body. This condition is extremely common in premenopausal women in industrialized countries, and greatly increases the risk of breast cancer.
Since most breast tumors are hormone-positive, correcting the hormonal balance can make a decisive contribution to reducing the incidence of the disease.
The level and duration of estrogen exposure are key factors that promote or prevent cancer. An association has long been observed between women's increased lifetime exposure to estradiol (early menarche, delayed menopause, parity zero) and an increased risk of developing breast cancer. Breast cells are highly estrogenic and estrogen is a conditional growth hormone in the body as it stimulates the division of estrogen sensitive cells *.
Estrogens and its quinone metabolites cause breast cancer through a two-layer mechanism in which they:
a) stimulate cell growth and division * * and
b) produce oxidative stress and DNA damage * * by increasing the rate of genetic mutations *.
Long-term exposure to androgen-unbalanced estrogen also induces phenotypic and genomic changes in mammary epithelial cells associated with highly proliferative, invasive, and migratory abilities * *.
Estrogens bind to the two main estrogen receptors in breast cells, ERα and ERβ. The binding of estrogen to the receptor causes the transcription of a large number of genes associated with it *. Both estrogen receptor subtypes are expressed in breast tissue, but estrogen-dependent cell proliferation and breast carcinogenesis are mainly associated with ERα signaling, while ERβ can downregulate ER-dependent gene transcription. However, the role of ERβ in breast cancer appears to depend on the presence or absence of ERα, as ERβ has been shown to stimulate cell proliferation in ERα-negative breast cancer cells *.
Progesterone is a female steroid hormone that balances the proliferative activity of estradiol. Their ratio in a woman of mature age is not constant; it fluctuates during the menstrual cycle, leaning one way or the other. Estradiol dominates in the first (ovulatory) phase of the cycle, stimulating cell growth, and progesterone dominates in the second (luteal) phase of the cycle, stimulating apoptosis and cell differentiation.
Both of these hormones are essential for breast development and actively promote cell division during breast formation as well as during pregnancy. Estrogen is thought to be the main stimulant for cell growth, but in fact, proliferation requires a combination of both of these antagonistic hormones *. While estrogen stimulates the growth of normal estrogen-responsive breast cells, progesterone can stimulate the growth of breast stem cells *.
Androgen is the general name for a group of steroid hormones produced in men by the testes and in women by the ovaries and adrenal cortex.
Testosterone is the main androgen in women. It is an anabolic hormone that is an estrogen antagonist. Although it is widely believed that estrogen is a female hormone and androgen is a male hormone, the balance between androgens and estrogens is important for both men and women. Hormonal imbalance is observed already at the initial stages of a tumor disease, which suggests a causal relationship between them.
An analysis of the hormonal profiles of patients with benign diseases in premenopause and postmenopause, found in them, on average, respectively, an increase in: estradiol – by 22.4% and 32.0%; estrone – by 26.2% and 30.9%; testosterone – by 19.5% and 16.5% compared with the profiles of healthy women *. The rate of proliferation directly depends on the level of these hormones in the circulating blood.
The concentrations of estrogen and androgen differ greatly in the tissues of benign and malignant tumors and in the circulating blood. In postmenopausal (but not premenopausal) breast cancer tissue, the levels of estradiol (by 56%), androstenedione (by 33%) and testosterone (by 33%) are significantly higher. And the levels of dehydroepiandrosterone and androstenediol are higher in benign than in cancerous tissues (respectively, 6 times and 2 times). Moreover, the concentration of androgens is higher in plasma than in tissues, which suggests their active transformation into estrogens by the adipose tissue of the mammary gland *. Partly due to this, the concentration of estradiol in a breast tumor in postmenopausal women can be 10-20 times greater than in plasma * *. Thus, if we draw conclusions based on the concentration of hormones in the blood, the true hormonal situation in breast tissue will elude us.
In premenopausal women, prospective studies show a direct relationship between circulating estrogen levels and breast cancer risk * *. Moreover, this risk is significantly associated with a high concentration of estradiol in the follicular, but not the luteal phase of the menstrual cycle *.
In postmenopausal women, the risk is higher * in groups with higher concentrations of total estradiol, free estradiol, estrone, and their precursors – androstenedione, dehydroepiandrosterone and testosterone * *. At the same time, higher levels of sex hormone-binding globulin (SHBG) are associated with lower risk. Any action that results in a decrease in circulating levels of estrogens, especially estradiol, has the potential to reduce the risk of breast cancer *.
Reducing the estrogen load can be achieved in the following ways:
1) removal of destroyers of the hormonal system;
2) improving the process of removing estrogen metabolites from the body;
3) improving the ratio of healthy estrogenic metabolites to carcinogenic metabolites;
4) decrease in the production of estradiol;
5) increase in the level of progesterone;
6) «silencing» the estrogen receptor;
7) control of thyroid receptors and receptors of other sex hormones.
Points 1, 2 and 3 are preventive; however, their value does not decrease during hormone therapy. Items 4 and 5 may be useful for high estradiol levels, but their use should be strictly controlled. The remaining items are purely therapeutic, and are extreme measures that should not be applied by women on their own.
The elimination of external hormonal disturbances is the first step in hormonal normalization *. Cancerogenic effects can have: natural or bioidentical estrogens, mainly estradiol (while estriol may have a protective effect); estrogen metabolites such as 4-OH-E2, 16α-OH-E2 and others; estrogen-like drugs such as oral contraceptives and hormonal drugs; xenoestrogens (herbicides, pesticides, fungicides, some plastics, including those used as packaging); many chemicals (refrigerants, industrial solvents, bleach by-products, polycyclic aromatic hydrocarbons, etc.), toxic metals (copper, cobalt, nickel, mercury, lead, tin and chromium *); hormones used for fattening animals and stimulating milk production that end up in the finished food product. Oral contraceptives or hormone replacement therapy alone can increase the risk of breast cancer by up to 25% *.
In 1996, the European Commission included xenoestrogens in the list of priority risk factors. Air, water and food pollution can occur due to the widespread use of xenoestrogens in agriculture and food production *, as well as the migration of phthalates into food from plastic packaging. Skin contamination with phthalates occurs when using deodorants, as well as cosmetic creams that contain phthalates and parabens. We can self-assess the skin care and household cleaning products you use at home for their xenoestrogenic risk using the independent online resource EWG *.
In addition to removing all of the above substances from our life as much as possible, we can additionally use detoxifiers to remove them from the body. Many hormone disruptors are fat soluble and can accumulate in body fat, and the breast is largely made up of fat. For example, tissue concentrations of bisphenol-A in cancer patients are significantly higher than in non-cancer patients (4.2 vs. 1.8 ng/g of tissue) *. Similarly, parabens accumulate * in breast tissues, and the same can be expected from other fat-soluble substances.
A significant contribution to the risk of developing breast cancer, due to the large amount of growth hormones they contain, is made by animal milk and products made from it, including cheeses and even yogurts *. Moreover, thermal and any other processing of milk does not lead to the destruction of insulin-like growth factor (IGF) *. Unfortunately, the harm of milk to the health of an adult is still underestimated in the mass consciousness.
Alcohol consumption, even in small doses, adversely affects breast health. For example, just 15 g/day of pure alcohol (a glass of wine) increases the serum estrone sulfate concentration by 7.5% *, thereby worsening the estrogen:androgen ratio. You should also completely eliminate the consumption of sugar and sugary foods and drinks.
Insulin regulators. While there are medical methods for lowering insulin, the most natural way to keep insulin levels low is to reduce total food intake and eat foods with a low glycemic load instead of foods with a high glycemic load. As an adjunct, if necessary, metformin can be used.
Estrogen regulators. Lowering total estrogen levels, especially estradiol, may reduce the risk of cancer in estrogen-sensitive tissues.
Because estrogens play a protective role for neurotransmitters, there are reasonable concerns that depleting the body of estrogens may adversely affect human cognitive function; however, this assumption is still debatable *.
Excess estrogen is bound (glucuronidated) by the liver and passed through the bile duct to the intestine for further removal. In the gut, this inactive estrogen can be released again and returned to the bloodstream, increasing the risk of cancer. This process is highly dependent on the composition of the food mass and, accordingly, on the composition of intestinal bacteria. Fiber and the gut bacteria that feed on it slow down and make it harder for estrogen to be absorbed back into the bloodstream. Thanks to them, excess estrogen is excreted from the body. In contrast, meat and the bacteria that feed on it can aid in the release and activation of estrogen.
In addition to increasing the risk of cancer, high estrogen levels can cause moderate to severe pain during your period. Estrogen stimulates the growth of hormone-sensitive cells in the uterus to prepare it to receive an egg. The more estrogen in the follicular phase, the more the uterine lining thickens. Accordingly, the more of this cell mass breaks down at the end of the cycle, into the luteal phase, and the more pro-inflammatory prostaglandins are released, which cause cramps and pain.
Some non-drug treatments can help lower overall estrogen levels.
• Lignans (from flaxseed) and fiber (from bran of cereals – wheat, rye, barley, oats) are prebiotics that create favorable conditions for the prosperity of those intestinal bacteria that have low activity of β-glucuronidase and β-glucosidase (for example, Lactobacillus acidophilus). A decrease in the activity of both of these enzymes improves the process of estrogen excretion through the intestines; increases the concentration of globulins that bind sex hormones (SHBG); reduces aromatase activity and overexpression of the HER2 oncogene; and generally improves patient survival *.
Women with high lignan intake, compared to women with low lignan intake, show a 44% lower risk of developing breast cancer * * *.
Postmenopausal breast cancer women with the highest lignan intake versus women with the lowest lignan intake show a 51% reduction in risk of all-cause mortality, and a 71% reduction in breast cancer mortality *.
Dosage: 25-30 g/day * finely ground flaxseed.
• Flaxseed oil contains ω-3 fatty acids, which reduce excessive levels of cholesterol and estrogen in the blood, especially estradiol (E2) and estrone (E1), shifting the estrogen balance in favor of the weaker estrogen, estriol (E3). Flaxseed oil helps to eliminate solid fats and dioxin from the body.
Each additional 0.1 g/day of ω-3 reduces the risk of developing breast cancer by 5% *. Patients with metastatic breast cancer on anthracycline-based chemotherapy can more than double survival with 1.8 g/day docosahexaenoic acid (DHA) *.
In addition, ω-3 fatty acids (4 g/day for 3 months) may offset the negative effects of aromatase inhibitors on bone tissue in postmenopausal women *. Dosage: 0.5-1 tablespoon/day.
Other plant sources that are slightly less rich in ω-3 fatty acids are False flax (Camelina) and Chia (Salvia hispanica) seed oils. In all these plants, the ratio ω-3:ω-6 in the oil is more than one, in contrast to other vegetable oils, where the ratio is reversed.
In the presence of oxygen, especially in the light, ω-3 fatty acids are rapidly oxidized, forming carcinogenic compounds. Therefore, vegetable oils should be taken fresh from the seller's refrigerator and stored in a home refrigerator for no more than a month.
• Fish oil is an alternative source of ω-3 fatty acids. Unlike cod liver oil, fish oil is found in fish tissue.
Whereas EPA and DHA are derived from flaxseed oil through the metabolism of alpha-linolenic acid (ALA), fish oil contains them in their finished form. This is especially important for people whose genetic characteristics do not allow efficient synthesis of these fatty acids from plant materials. And there are quite a lot of those among the European population.
In addition, the metabolic efficiency of vegetable ω-3 is very low. The conversion of ALA to EPA and EPA to DHA is estimated to range from 5-10% and 2-5% respectively *. Thus, fish ω-3s are more effective than plant ω-3s *. However, for long-term storage, fish oil is recommended to be purchased in sealed gelatin capsules due to its high oxidizability. Dosage: up to 1 ml/day *.
• D-glucarate is found in grapefruit, apples, oranges, broccoli, and Brussels sprouts. It inhibits the activity of β-glucuronidase; contributes to the safe metabolism, binding and removal of estrogens from the blood and their transformation into a less toxic form *. D-glucarate reduces systemic inflammation and significantly inhibits the growth of grafted mammary tumors in animals * *. The combination of glucarate with low doses of retinoids works synergistically in animal studies *. Dosage of calcium D-glucarate: 1'200-3'000 mg/day *.
• Soy milk consumption for 3 months (400 ml/day, ~ 109 mg/day of isoflavones) reduced estrone and estradiol levels in Japanese women by 23% and 27%, respectively *. A similar decrease in estrogen levels has been noted in men, with no effect on androgens observed *.
In American premenopausal women, consumption of 1 liter of soy milk per day (~ 200 mg of isoflavones) for a month reduces the level of estradiol in the follicular phase by 31%, and in the luteal phase by 49%. At the same time, the level of progesterone in the luteal phase decreased by only 35% *. Interestingly, even after stopping soy milk, estrogen levels remained low for 2-3 monthly cycles.
The consumption of soy and related soy isoflavones may have a beneficial effect on the menstrual cycle in young women (20-30 years old). In one study, consumption of 60 g/day of soy protein (45 mg/day of isoflavones) significantly extended the menstrual cycle by lengthening the follicular phase *. The highest level of breast epithelial cell growth occurs during the luteal phase, so prolonging the follicular phase of the cycle shortens the duration of the luteal phase, and thus may reduce the risk of hormone-sensitive cancers. However, not all studies have shown such high success rates for soy products.
• Iodine seems to be able to desensitize breast cells to estradiol *.
Lifestyle changes can have a greater effect on lowering estrogen levels than supplementation without any negative effects. And this applies to women of any age and any status of menopause.
A moderate decrease in fat intake during puberty is associated with positive changes in the concentrations and ratios of sex hormones; for example, estrogen levels can be reduced by up to 30% *. A diet with a very low fat content (10-12% of the total caloric intake of food) and a high fiber content (25 g/day) within 2 months significantly reduces the level of estradiol and estrone in healthy women, without affecting ovulation in any way * * *. However, as the fat content of the food is increased above 20% of the total calorie content, this effect is weakened *.
Reducing the contribution of fat to calorie intake from 40% to 20% also significantly reduces plasma hormone concentrations in healthy postmenopausal women *. As early as 14 weeks after reducing fat intake from 68.5 g/day to 29.5 g/day, plasma estradiol levels fall by 17% *.
In one manipulative study, postmenopausal women, both with and without hormone replacement therapy, were switched to a 3-week low-fat (10% of total calories) and high-fiber (65-70 g/day) diet at combined with exercise. As a result, SHBG (sex hormone-binding globulin) levels increased by a quarter, insulin levels decreased by a third, and body mass index and total cholesterol also dropped significantly *. This regimen significantly reduces the level of growth factor IGF-1, and increases the level of IGF-1 binding protein. Blood taken from women after 2 weeks of the experiment, in vitro reduced growth by 7-18% and increased apoptosis of cells of various breast cancer lines by 20-30% *.
Vegetarians, compared with omnivores, show significantly lower plasma levels of estradiol, free estradiol, free testosterone, and significantly higher levels of SHBG *. Removing any type of alcohol from our diet reduces the levels of circulating steroid hormones, especially estrogen *. A decrease in body fat mass will also play a role in reducing estrogen, because adipose tissue is a factory of estrogen.
The reduction in estrogen activity can be achieved in several ways. For example, by converting a more active estrogen (estradiol) into a less active one (estrone). This can be achieved, on the one hand, by suppressing HSD17β1 dehydrogenase with specific inhibitors *, and on the other hand, by activating HSD17β2 dehydrogenase, for example, with retinoic acid *.
Several flavonoids have an in vitro weak inhibitory effect on 17HSDβ1 activity (genistein, lignans *, gossypol *, diosmetin, scutelarin, fisetin *, chrysin, kaempferol *, quercetin, myricetin, naringenin, luteolin, biochanin A *). A shift in the ratio between E1, E2 and E3 in favor of E1, and especially in favor of E2, called estrogen imbalance, is very common throughout the world. And any manipulations aimed at correcting it could reduce the estrogen load.
Even more reduces the activity of estrogen, its translation into an inactive state by chemical reactions of glucuronidation and sulfation, as well as its binding to special globulins, which are called sex hormone-binding globulins (SHBG). Doubling the serum concentration of these globulins is associated with a 22% reduction in cancer risk *.
Steroid sulfatase inhibitors block the reduction of bound estrogen to its active form. Currently, several specific irreversible steroid sulfatase inhibitors have been developed.
A diet with no calorie restriction but low in animal fats and refined carbohydrates, rich in low glycemic index foods, monounsaturated and ω-3 polyunsaturated fatty acids and phytoestrogens, can beneficially alter the hormonal profile of postmenopausal women *. In the intervention group, compared with the control group, sex hormone-binding globulin levels increased significantly and serum testosterone levels decreased.
Switching to a traditional «Mediterranean» diet (from the predominant intake of animal fats and proteins to the predominant intake of vegetable fats and proteins) within 6 months significantly (more than 40%) reduces estrogen levels *. Much of this effect was based on a marked reduction in specific estrogen metabolites, including the hydroxy and keto derivatives of estradiol or estrone.
Aromatase activity inhibitors. The concentration of estrogen can be reduced by suppressing its synthesis. Aromatase is an enzyme involved in the conversion of androgens to estrogens. The increase in aromatase activity contributes significantly to the increase in estrogen and increases the imbalance between androgen and estrogen, thereby stimulating tumor growth.
Known factors that increase aromatase activity include age, inflammation, obesity, alcohol, insulin, and gonadotropins. Research has also established the presence of an obesity-inflammation link that stimulates aromatase activity * in breast tissue and is likely associated with an increased risk of hormone-sensitive breast cancer subtypes.
Suppression of peripheral and interstitial * estrogen activity with aromatase inhibitors helps to reduce the risk of cancer and its recurrence *. Aromatase inhibitors are comparable in their therapeutic effect to inhibiting tumor growth to the removal of the sex hormone-producing ovaries *.
Two main classes of aromatase inhibitors are currently in clinical use. Type I steroid preparations include formestane and exemestane; they are analogous to an androgenic substrate that binds competitively but irreversibly to the enzyme. Non-steroidal type II inhibitors such as anastrozole and letrozole bind to the enzyme reversibly and provide exceptional potency and specificity against aromatase.
Anastrozole * and exemestane * more than halved the incidence of ER+-subtypes breast cancer. With exemestane, no side effects, such as cognitive decline, have been reported that are commonly associated with tamoxifen treatment. Other next-generation aromatase inhibitors with milder negative side effects are being developed. Norendoxifen, a metabolite of tamoxifen, acts as a strong competitive aromatase inhibitor (IC50 = 90 nM) and may also be involved in its antiestrogenic activity *.
Some non-prescription aromatase inhibitors are used in bodybuilding.
ATD (androstatrienedione) is very popular because it is cheap and effective, but it can reduce libido. Methylated ATD is preferred because it is non-toxic to the liver. Dosage: up to 75 mg/day.
3-OHAT is just as effective as ATD and is faster acting than ATD.
Formestane, aka 4-OHAD, can be used transdermally, which will require not only a lower dose, but also less frequent dosing.
6-OXO, an irreversible inhibitor, is also popular. Consumption of 300mg/day of 6-OXO for 8 weeks is able to increase free testosterone by 90%, DHT by 192%, estrone by 22%, and testosterone to estrogen ratio by 53% from baseline *.
The disadvantages of synthetic aromatase inhibitors, in addition to their side effects, include the ability of cells to quickly develop drug resistance to them *, which causes relapses of the disease * and re-growth of the tumor after 12-18 months of treatment. Inhibitors of MEK, Raf, PI3K, mTOR and Akt * are considered as possible candidates to overcome drug resistance. Unfortunately, tumors are able to enhance non-traditional pathways of sex hormone conversion, bypassing the inhibited traditional pathways *.
Do not forget about the mutual action of various receptors and hormones. Blocking the conversion of androstenedione to estrone may increase its conversion to testosterone. Therefore, inhibition of aromatase without simultaneous inhibition of aldoketoreductase can create conditions for increasing the load on the androgen receptor. Which in triple negative breast cancer is likely to be counterproductive.
Natural aromatase inhibitors cannot compete in effectiveness with synthetic ones, but they do not have such strong side effects. However, both individually and as part of complexes, they can enhance the effect of aromatase inhibitors prescribed by a doctor and, due to this, reduce their dosage and side effects. Many natural aromatase inhibitors are found in foods, which gives us some opportunity to influence aromatase activity through dietary modification.
At the moment, about 300 natural products have been evaluated for their ability to inhibit aromatase, most of which are so-called phytoestrogens; however, only a few positive research results have been published *.
• Polyphenols.
Flavonoids that showed the most pronounced antiaromatase effect in vitro *: chrysin, contained in the leaves of Passiflora (Passiflora), 500 mg/day; apigenin, contained in the root of Sage (Salvia officinalis); 8-prenylnaringenin, contained in Hops cones (Humulus lupulus); quercetin from the leaves of Ginkgo (Ginkgo biloba); biochanin A from the leaves of Red clover (Trifolium pratense).
Biochanin exhibits a dual action: it inhibits aromatase activity at low concentrations, but exhibits estrogenicity at high concentrations *. Biochanin is effective in reducing the growth of estrogen-dependent MCF-7 tumors at equiv. dose of 1 mg/kg per day *.
Kaempferol, that found in ginkgo leaves, is also known as an aromatase inhibitor that enhances the effects of tamoxifen *. Plant extract (500 mg/kg) strongly inhibits aromatase activity and reduces in vitro and in vivo estradiol content in ER+-subtype breast cancer cells with overexpression of aromatase (AROM MCF-7) *. One of the pharmacy options for ginkgo is Tanakan™ (leaf extract). Dosage: 40 mg of dry extract *, or 1 ml of 10% alcoholic extract of plant leaves three times a day.
In an in vitro study on ER+-subtype breast cancer cells (MCF-7), flavonoids inhibited the activity of aromatase enzymes in the following order of decreasing effect: chrysin → naringenin → genistein → apigenin → biochanin → quercetin. However, it was noted that the estrogenicity of these phytochemicals was more pronounced than their inhibition of aromatase *. In addition, a common disadvantage of flavonoids is their poor bioavailability, which significantly reduces their effect in vivo.
Chalcons. 8-isoliquiritigenin contained in the root of Licorice (Glycyrrhiza glabra) at a concentration of 36 µM reduces the in vitro expression of genes associated with aromatase, suppressing the production of estrogen *. Unlike many other aromatase inhibitors, which show a dual effect depending on the concentration, isoliquiritigenin does not show estrogenicity in vivo, and does not affect the weight of the uterus in experimental mice *. Dosage isoliquiritigenin: equiv. 800 mg/day *.
Lignans such as enterodiol and enterolactone from flax seeds and stilbenes like resveratrol from grape skins * are also natural aromatase inhibitors.
• Myrrh resin (Commiphora myrrha) exhibits a strong anti-aromatase effect in vitro due to the triterpenes it contains *.
• Mushrooms – Oyster mushroom (Pleurotus), Champignon (Agaricus bisporus), and some others, inhibit aromatase activity * and can reduce the incidence of breast cancer by 50%, and in combination with green tea – by 90% *. Mushroom ethyl acetate extract contains conjugated linoleic acid, which is able to block aromatase activity. Dosage: 100 g ground on a blender and boiled mushrooms *.
• Fresh juices (in descending order of anti-aromatase effectiveness: pomegranate, red grape, strawberry, apple, plum, grapefruit, peach, orange). Dosage: 250 ml/day.
Grape (Vitis), red wine or grape seed extract rich in procyanidins and flavanoids, which have an anti-aromatase effect. Red wine increases free testosterone levels and decreases SHBG levels with a very slight decrease in estradiol levels *. The most useful wine varieties are Merlot * *, Pinot Noir, Cabernet, Chardonnay *. To reduce the negative role of alcohol, before use, it can be partially evaporated by a short heating. Dosage: 100 mg of extract or 125 ml of dry red wine per day *.
Pomegranate (Punica granatum), fermented juice (wine), seed oil and pericarp ethanolic extract. The ellagic acid contained in the pomegranate, the ellagitannins that metabolize to ellagic acid, and especially urolithin B, inhibit in vitro the growth of breast cancer cells *, especially estrogen-dependent *, and inhibit aromatase * *. Pomegranate red wine contains 3 times more antioxidants than grape red wine *.
• Other most commonly mentioned natural aromatase inhibitors are sodium butyrate, selenium, zinc, magnesium, vitamin D *.
Melatonin * * can also act as an aromatase inhibitor in ER+ cells (T47D), showing activity comparable to letrozole, but without the side effects of the latter. Dosage: 20-40 mg/day * *.
Metformin also appears to have some anti-aromatase effect as it activates AMPK, the enzyme responsible for estrogen biosynthesis in human breast fat cells * *.
Sildenafil (Viagra™), tadalafil and vardenafil, used to treat erectile dysfunction, are able to effectively (up to 35%) suppress estrogen biosynthesis *.
Unfortunately, oral aromatase inhibitors may only be therapeutically effective in postmenopausal or ovariectomized women. The reason is that in premenopausal women, aromatase inhibitors, by lowering estradiol levels, stimulate the release of gonadotropins from the hypothalamus, and gonadotropins signal the ovaries to produce estrogens in order to restore their natural levels *. As a result, the direct effect of aromatase inhibitors will be suppressed, and side effects, including weakening of the bone and the risk of ovarian cysts, will appear *. Topical application of aromatase inhibitors will probably have a greater effect than their systemic application.
There is another problem with the use of aromatase inhibitors. Recently, androgenic metabolites such as 5α-androstane-3β, 17β-diol (3β-diol), androst-5-ene-3β, and 17β-diol (A-diol) produced by other enzymes have been identified that also activate the estrogen receptor (ERα) *. Using these metabolic pathways as an alternative to aromatase, cells are able to develop resistance to aromatase inhibitors. There are probably other as yet unknown workarounds for converting androgens to estrogens.
Regulators of estrogen metabolism. Estrogen hormones are excreted from the body in urine and faeces after their metabolic conversion into less bioactive and more water soluble compounds.
The main metabolites of estradiol and estrone are their hydroxylated forms – 2-OH and 16α-OH, other metabolites are present in relatively smaller amounts. Of these, 16α-OH has the most pronounced estrogenic activity, which may exceed the activity of the hormones themselves * *. 16α-OH-E1 production is increased in a woman with breast cancer or at high risk of developing breast cancer.
Metabolites of 2-OH, on the contrary, have some antiestrogenic effect. Low levels of 2-hydroxyestrone have been associated with a higher risk of breast cancer in premenopausal women *. Because 2-OH-E2 is methylated faster than 4-OH-E2, the latter accumulates in the breast to a greater extent than 2-OH-E2 *.
4-OH metabolites damage protein structures, including DNA, contributing to both the appearance of cancer and its resistance.
Regardless of the absolute level of estrogens, it is important to reduce the ratio between stronger and weaker estrogens (and their metabolites). Those. reduce the ratio of estradiol to estrone and estriol; 2-OH-estrone to 4-OH-estrone and 16α-OH-estrone; 2-OH-estriol to 4-OH-estriol and 16α-OH-estriol.
Dietary changes and supplements can significantly shift the balance between «bad» and «good» estrogen metabolites in favor of the latter.
• Diet. Reducing meat consumption and increasing fiber intake reduces the concentration of conjugated steroid hormones in the intestine; increases the blood level of globulin that binds sex hormones, limiting the bioavailability of estrogens; and removes bile toxins from the body.
• Flaxseed, consumed freshly ground, increases the ratio of 2-OH-E1:16α-OH-E1 by improving the intestinal microflora. Transferring mice to a diet with 10% flaxseed of the total food volume inhibited the growth rate of the grafted ER– mammary tumor (MDA-MB-435) by 45%, and reduced its metastasis rates by 2.5 times *. In ER+ tumor-grafted mice (MCF-7), flaxseed consumption also retarded tumor growth and significantly enhanced the antiproliferative effect of tamoxifen * *. Regular consumption of ground flaxseed significantly increases serum 2-OH-E1 levels and 2-OH-E1:16α-OH-E1 ratio in postmenopausal women * * *, reducing the risk of premenopausal breast cancer *.
Dosage: 15 g/day *.
• Indole-3-carbinol (I3C) increases estrogen 2-hydroxylation * * and shows a statistically significant regression of uterine cancer within 12 weeks of supplementation *. Dosage: 400 mg *.
• Diindolylmethane (DIM), an indole-3-carbinol dimer, also increases the 2-OH-E:16-OH-E1 ratio * but reduces plasma levels of tamoxifen metabolites *. Dose: 2x150mg BioResponse DIM® *.
• Omega-3 fatty acids, dietary fiber, cimetidine, progesterone also improve the 2-OH:16α-OH ratio.
• Resveratrol at a dosage of 1'000 mg/day on average by 73% increases the content in the urine of 2-hydroxyestrone (2-OH-E1) in postmenopausal women, which leads to a favorable change in the ratio of 2-OH:16α- OH-E1 *.
• Genistein, contained in soy, hydroxylates estrogens into less mutagenic and carcinogenic metabolites – 4-OH-E2 and 4-MeO-E1 *. Genistein also increases the concentration of sex hormone-binding globulins (SHBG) in the blood, which reduces the level of free active estrogens and androgens. Dosage of genistein: 150 mg/day during treatment and 40 mg/day in prophylactic regimen. Meanwhile, consumption of whole soybeans is likely to provide more positive effects than consumption of one of its components.
• Bioactive substances isolated from Black cohosh (Actaea racemosa) and Red clover (Trifolium pratense) enhance the activity and expression of mRNA of the CYP1A1 enzyme, which is responsible for the conversion of estrogens into non-carcinogenic metabolites 2-OH-E1 and 2-OH-E2 * *. Contrary to fears, the results of many studies show that Black cohosh does not promote the growth of breast cancer cells, both in vitro and in vivo.
• Licorice (Glycyrrhiza sp.), an aqueous infusion of the root. In mammary gland epithelial cells, various types of licorice, Glycyrrhiza glabra (GG) and Glycyrrhiza inflata (GI) and the compounds contained in them differently modulate the expression of the CYP1A1 gene responsible for estrogen detoxification and the CYP1B1 gene responsible for genotoxicity. GG and GG-containing glycyrrhetinic acid * and isoliquiritigenin increased CYP1A1 expression, while GI and licochalcon A decreased CYP1A1 and CYP1B1 expression *.
• 6-prenylnarnigenin from hop extract attenuates estrogen-induced CYP1B1 expression *, thereby enhancing non-toxic estrogen 2-hydroxylation. Dosage of hops ethanol extract: 300 mg/day.
• Pomegranate polyphenols at concentrations of 100-1'000 μg/mL in vitro inhibit the estrogen enzyme 17-beta-hydroxysteroid dehydrogenase 1 (HSD17B1) from 34% to 79% in the following order of effectiveness: seed oil → fermented juice polyphenols → pericarp polyphenols *. The combined use of ellagic acid with phosphatidylcholine in a ratio of 1:4 can double its bioavailability *.
• Retinoic acid (vitamin A) in vitro through the activity of retinoic acid receptors induces the expression of antiestrogen enzyme 17-beta-hydroxysteroid dehydrogenase 2 (HSD17B2), which transforms highly active estrogen (estradiol) into weakly active (estrone) *, which can reduce the risk of cancer of the uterus and ovaries.
• N-acetylcysteine and resveratrol in vitro prevent the oxidation of estrogen to metabolites that cause breast cancer * * *.
• Vitamin B6 in vitro detoxifies excess estrogen through methylation, reducing the risk of estrogen-dependent cancer *. An increase in the level of intracellular B6 leads to a weakening of the transcriptional response to glucocorticoids, progesterone, androgen or estrogens. Conversely, cells deficient in vitamin B6 show increased sensitivity to steroid hormones * *.
• Vitamin C plays an important role in hormone synthesis *. Oral intake of 1 g/day of vitamin C increases the level of estradiol, depending on the initial level of the vitamin in the body, by 20-50% *. And at the same time, it reduces the production of aromatase in the ovaries *.
• Vitamin D3 increases the in vitro synthesis of progesterone, estradiol and estrone *, while it reduces the expression of aromatase and inflammatory cytokines in macrophages *, and enhances bone protection when estrogen levels are reduced *.
• Vitamin Е promotes estrogen detoxification. Some (but not all) forms of vitamin E inhibit the action of estrogen, especially in breast tissue. The action of γ-tocotrienol in ER+ MCF-7 breast cancer cells is enhanced when it is combined with EGCG and resveratrol *. Cell proliferation in the presence of EGCG (50 µM), resveratrol (25 µM), and γ-tocotrienol (10 µM) was about 33%, 50%, and 58%, respectively, against control. Feeding rats for 9 weeks with mixed γ- and δ-tocopherols in an amount of 0.1%, 0.3% or 0.5% of the total food volume inhibited the growth of a mammary tumor in them by 38%, 50% or 80%, respectively *.
• Vitamin K2 in vitro helps reduce the estradiol:estrone ratio by binding to the estrogen enzyme 17-beta-hydroxysteroid dehydrogenase 4 (HSD17B4), which converts estrone to estradiol *, and inhibit estrogen activity by binding to its receptor *. Unfortunately, high doses of vitamin K can promote blood clots.
• Iodine in the form of iodized salt is able to change the expression of at least 43 different genes involved in hormone metabolism, and also involved in the regulation of the cell cycle, growth and differentiation of breast cells *.
• Exercise and a high protein diet can also help lower the 2-OH:16α-OH.
Another option for the safe metabolism of estrogens is their glucuronidation and sulfation, as a result of which they lose their biological activity. Currently known agents that promote estrogen sulfation are celecoxib, resveratrol, quercetin, glucocorticoids.
Targets to strive for by regulating estrogen metabolism:
- the level of 16α-hydroxyestrone in healthy women: 10-15 ng/mL in premenopausal women, and 4 ng/mL in postmenopausal women;
- the level of 2-hydroxyestrone in healthy women: 13-23 ng/mL in premenopausal women, and 6 ng/mL in postmenopausal women;
- the ratio of estrogen metabolites 2-OH-E1 to 2-OMe-E1: 0.6-6 (in healthy pre- and postmenopausal women);
- the ratio of estrogen metabolites 2-OH-E1 to 16α-OH-E1: from 1.6 in premenopause to 1.5 in postmenopause;
- the ratio of estrogen metabolites 4-OH-E1 to 4-OMe-E1: from 0.4 in premenopause to 0.3 in postmenopause.
Estrogen receptor modulators. Estrogens promote the growth of breast cancer through estrogen receptor alpha (ERα) signaling, which is expressed in approximately 70% of breast cancers *. Therefore, another anti-estrogen control strategy, in addition to lowering estrogen levels, is to reduce the activity of the estrogen receptor (ER) in estrogen-responsive cells. However, it has been reported that such therapy may be less effective than aromatase inhibitors *.
Selective Estrogen Receptor Modulators (SERMs) such as tamoxifen or raloxifen are effective against recurrence of ER+ breast tumors *. Tamoxifen is metabolized in the liver by the enzymes CYP2D6 and CYP3A4. The resulting metabolites act as an ER antagonist, competing with estrogens for estrogen receptors in breast tissue cells and inhibiting the transcription of estrogen-responsive genes *. Tamoxifen metabolites show a greater affinity for ERα and ERβ than estradiol: 1.8 and 3.4 times, respectively *. Because ERα and ERβ receptors are expressed to varying degrees in different tissues, tamoxifen acts as an ERα antagonist in the mammary gland and as an ERβ agonist in the uterus and ovaries *, increasing the risk of cancer in these organs.
Over-the-counter estrogen receptor modulators are less effective, but they are safer and may increase the effect of SERMs prescribed by a doctor, thereby allowing a reduction in the dosage of the latter. They can also be used in place of prescription SERMs after they have been discontinued.
• Garlic (Allium sativum), especially enriched in selenium *, inhibits in vitro activity of the estrogen receptor ERα in breast cancer cells. Diallyl trisulfide (DATS), a component of garlic oil, at a concentration of 20 µM reduces the vitality of ER+ (MCF-7 and T47D) cancer cells by about a quarter *. Unlike some other therapeutic agents, the inhibition of cell growth induced by DATS was not abolished by overexpression of estradiol or ERα. Taking garlic can significantly reduce the development of already diagnosed stage II and III breast cancer *. Intraperitoneal injections of diallyl trisulphide contained in garlic oil into mice reduced the rate of cancer development by a factor of three *. Dosage: 200mg DATS in combination with 100 μg selenium *.
Alternative sources of DATS are aged garlic (aged 10 months after being minced and mixed with an equal amount of a water-alcohol solution *); black garlic (garlic fermented for 2-4 weeks at 60-90 °C and 80-90% humidity); dry garlic powder. The content of DATS in garlic is about 1-3%, so the concentrations needed to suppress ER expression and function can be achieved by consuming 10-15 g/day of raw garlic *. Raw garlic phytoncides are unlikely to make such a high garlic intake possible. They have a burning effect on the mucous surface of the gastrointestinal tract, destroy the intestinal flora and cause bad breath. At the same time, the garlic product taken in enteric capsules is free from these disadvantages. Dosage: 10 g/day raw garlic *, or 500 mg dry aged garlic extract *.
• Selenium reduces ERα protein expression levels and reduces estradiol binding to the estrogen receptor in ER+ breast cancer cells (MCF-7) in vitro, while increasing ERβ protein expression in ER-breast cancer cells (MDA-MB-231) *. In mice inoculated with an ER+ mammary tumor (MCF-7), methylselenocysteine synergistically enhances the antitumor efficacy of tamoxifen *. And in mice grafted with triple-negative breast cancer (MDA-MB-231), methylselenic acid synergistically enhances the efficacy of paclitaxel *. Dosage of methylselenocysteine: up to 7 mg/day *.
• Hops (Humulus lupulus) contains 8-prenylnaringenin, which exhibits binding to ERα * many times stronger than any other phytoestrogen *. By competing with estrogen for ER, it can reduce the estrogenic effect of the latter *. In addition, hop extract and 8-prenylnaringenin prevent malignant transformation of the breast *. Low doses of hop extract have no estrogenic effects on the uterus *. Dosage: Three times a day 500 mg of hop tablets containing 0.1 mg of 8-prenylnaringenin *.
• Indole-3-carbinol (I3C), found in cruciferous plants, has a low concentration proliferative effect in ER+ breast cancer cells (MCF-7) in vitro * * *. And at a high concentration (100 µM), it has an antiproliferative effect *, mainly due to a decrease in the expression of ERα mRNA *. Unlike tamoxifen, I3C does not compete with estrogen for binding to the receptor, but uses different mechanisms (ligand binding to AHR), and due to this, it can enhance the in vitro antiproliferative effect of tamoxifen *. The combination of I3C and genistein synergistically enhances the expression of GADD (DNA damage indicator protein), thereby increasing apoptosis, and allows a reduction in the effective dose of each of these phytochemicals *. Dosage: 400 mg/day *.
• Ellagic acid * * and isomers of linoleic acid * *, contained in the fruits of Pomegranate (Punica granatum), in vitro impair the binding of estradiol (E2) to ERα and ERβ cell receptors, inhibiting the growth and proliferation of ER+ breast cancer cells. In addition, an emulsion of a mixture of fermented juice and pomegranate seed oil * reduces the value of the expression ratio of ERα:ERβ, which was found in experiments on rats at a dosage of 5 mg/kg *. Although pomegranate extract acts similarly to tamoxifen and other SERMs, it does not appear to increase cell proliferation and uterine mass in ovariectomized mice *.
Pomegranate fruit extract (300 µg/mL) in vitro enhances tamoxifen-induced estrogen inhibition, cell cycle arrest, and apoptosis in breast cancer cells *. In addition, it restores tamoxifen sensitivity in tamoxifen-resistant tumor cells. The polyphenols of fermented juice (wine) show about twice the antiproliferative effect compared to the polyphenols of fresh pomegranate juice. Pomegranate seed oil (100 μg/mL) in vitro resulted in a 75-90% suppression of proliferation in ER+ breast cells (MCF-7), and in 54% of ER– cells (MDA-MB-435) caused apoptosis *.
Dosage: equiv. 500 mg/day pomegranate methanol extract *. In addition to its effect on estrogen, one glass of pomegranate juice a day can increase serum testosterone levels by an average of 24% in both men and women in 2 weeks *. However, in postmenopausal women, a daily intake of 250 g of juice can cause a significant decrease in estrone and testosterone levels in women *.
• Epigallocatechin gallate (EGCG) and other green tea polyphenols increase the in vitro efficacy of tamoxifen in ER– breast cancer without altering ER protein expression *. EGCG treatment inhibited the invasive ERα+ phenotype of mammary tumor cells in mice *. In triple negative breast cancer implanted mice, the combination of EGCG (25mg/kg) with tamoxifen (75 μg/kg) reduced tumor volume growth by 71% compared to untreated controls *. However, six-month studies of women with stage I or II ER– breast cancer who took 400-600 mg/day of EGCG showed a very slight decrease in ERα * expression. Dosage of EGCG: up to 1'200 mg/day * *, equivalent to 12-18 cups of green tea per day.
• Baicalein, a flavonoid from Baikal skullcap (Scutellaria baicalensis) root, can bind estrogen receptor α (ERα) and G protein-coupled estrogen receptor (GPER), which are two critical pathways associated with estradiol *. This compares favorably with tamoxifen, which is an ER antagonist but a GPER agonist, thereby increasing the risk of uterine and ovarian cancer in women taking tamoxifen. Baicalein dosage equivalent to that studied in animals *: up to 125 mg/day, this amount is contained in approximately 20 g of dry root.
• Resveratrol, quercetin and catechin, the polyphenols found in grape skins, also act as selective estrogen receptor modulators *. Polyphenols, however, show orders of magnitude less activity than natural hormones. Resveratrol, for example, binds ERα and ERβ 7'000 times weaker than estradiol (E2) *. Feeding mice with a mixture of equal parts of reveratrol, quercetin and catechin at a total dosage of 5 mg/kg three times a day reduced the size of grafted TNBC tumors (MDA-MB-231 and MDA-MB-435) by a third in comparison with 77 days of the experiment with control *, and the most significant contribution was made to resveratrol and quercetin. Due to the negative impact of alcohol on breast health, it should be removed from red wine before drinking; at home, this can be done by evaporation.
• Beta-sitosterol is an estrogen receptor agonist that preferentially binds to ERβ *. Small supplements of beta-sitosterol in the diet of mice (1% of food weight) reduce their serum estradiol levels by a third, but its excessive consumption from food or supplements can have an estrogenic and proliferative effect on ER+ breast cancer cells. In addition to regulating hormones, beta-sitosterol lowers blood cholesterol (LDL), sugar and estrogen levels, suppresses inflammation and boosts immunity *. Dosage: 330-500 mg/day.
• Ginkgetin, a biflavonoid from the leaves of Ginkgo (Ginkgo biloba), in vitro induces the death of ER-positive breast cancer cells by inhibiting estrogen receptor expression *.
• Ru-Pi-Xiao, a traditional Chinese collection (6 g/day) used for breast hyperplasia. It helps to increase progesterone levels naturally, without exogenous additives, and thereby enhance the effectiveness of tamoxifen (20 mg/day) *. The duration of admission is at least 3 months.
• Melatonin in vitro reduces the size and number of mammospheres in breast cancer cells by blocking the binding of estrogen to the receptor in ER+ cancer cells *, including cancer stem cells * *. Melatonin exhibits a dual antiestrogenic effect: not only does it block the metabolism of estrogen into more active forms and keep circulating estrogen inactive * *, but it also desensitizes estrogen receptors, reducing cell growth stimulation * *.
The combination of melatonin with tamoxifen enhances tamoxifen's in vitro suppression of breast cancer cell growth by a factor of 100 *. Taking 20 mg of melatonin at night and 20 mg of tamoxifen at noon for 8 months produced a partial response in 28.5% of patients with metastatic breast cancer who did not respond to pre-treatment with tamoxifen *. In another study, in a group of 25 hopeless cancer patients who had not responded to all previous treatments, the same treatment regimen produced a partial response in 3 and stabilized another 13 *.
Several naturally occurring substances, including morine, silybin, epigallocatechin gallate (EGCG), myricetin, baicalein, curcumin, kaempferol, or quercetin, have been reported to increase the bioavailability of tamoxifen and its metabolites *.
Unlike aromatase inhibitors, estrogen receptor suppression is effective in both premenopausal and postmenopausal women, however, a number of negative side effects due to the use of ER inhibitors should be considered:
• In 16% of women taking tamoxifen, atypical hyperplasia *, prone to neoplasia *, is observed in the uterus and ovaries. Progesterone remains the main means preventing this, and genistein (50 mg/day, at least six months) and metformin (850 mg/day) are alternatives * *.
• Women taking tamoxifen are up to 5 times more likely than untreated women to develop ER– tumors in the contralateral breast *, which are more aggressive than ER+ tumors. This effect can be countered by a combination of high physical activity and epigenetic agents such as green tea extract and broccoli extract, as well as other cruciferous vegetables such as kohlrabi, Brussels sprouts, cauliflower, mustard greens, swede, radish, wasabi/horseradish *.
• A decrease in estrogen levels can lead to a weakening of bone mass, which can be compensated to some extent by taking ω-3 fatty acids, vitamin D, cordyceps (5-20 ml/day), melatonin (3-20 mg/day) *, as well as complex of calcium, magnesium and phosphorus *.
• Another negative effect of tamoxifen and raloxifene is the so-called «thickening» of blood and increased thrombus formation *. To compensate for these phenomena, the use of drugs such as bromelain, aspirin, as well as limiting the intake of vitamin K may be indicated.
• Tamoxifen significantly reduces the sensitivity of cells to insulin, predisposing to type II diabetes. This effect can be compensated to some extent by taking metformin (500 mg/day).
• Loss of estrogen results in a decrease in plasma levels of copper, chromium, zinc and magnesium *. Thus, in postmenopausal women who have undergone spaying, or who are taking estrogen inhibitors, and who are not eating enough vegetables and whole grains, it may be necessary to take these elements in the form of supplements.
Patients who are prescribed tamoxifen can discuss with their doctor the possibility of changing it to raloxifene *, and even better – toremifene or lasofoxifene, which show a significantly lower carcinogenic effect on the uterus and ovaries, as well as a significantly lower risk of stroke, cataracts and thrombosis/thromboembolism *. On the other hand, tamoxifen is more effective * and retains its effect for 5 years after stopping it. While the effect of other SERMs may decrease over time *.
An alternative to oral tamoxifen is the proprietary gel BHR-700 (Besins Healthcare), which contains 4-hydroxy tamoxifen (4-OHT) as the active ingredient. Unlike the tablet form, tamoxifen gel can be easily absorbed through the skin of the breast and enter directly into the mammary gland, which reduces its dosage and side effects; including the uterus. However, it has not yet passed clinical trials and has not received FDA approval.
Another alternative would be to use the natural hormone estriol instead of tamoxifen. Estriol exhibits a 10 times weaker estrogenic effect than estradiol. Thus, by competing with estradiol for the same receptor, estriol could reduce the proliferative activity of estradiol. But such a therapy is still at the stage of a bold proposal.
Another, more affordable and proven improvement in hormone therapy is the addition of progesterone, a natural androgen, to tamoxifen. In patients with advanced postmenopausal breast cancer, alternating weekly tamoxifen (20 mg/day) with tamoxifen+progesterone (500 mg/day oral progesterone) doubled the efficacy of tamoxifen in terms of tumor shrinkage, disease stabilization, and time to before it progresses *.
Due to the increased risk of genital cancer, which is usually diagnosed at an advanced stage, regular screening is recommended for women taking SERMs.
GPER receptor modulators (G protein-coupled estrogen receptor 1). GPER is the third recently discovered estrogen receptor and, along with ERα and ERβ, is considered to be an important modulator of tumor development *. Unlike ERα and ERβ, which act through the regulation of gene expression, GPER acts more rapidly, in a non-genomic way, by activating signaling pathways involved in cancer cell survival, proliferation, and invasion. GPER-1 is widely expressed in breast cancer cell lines and primary breast tumors * *.
Whether this receptor should be activated or down-regulated remains a hotly debated topic due to conflicting research results. In different tissues and under different conditions (in particular, the presence or absence of its ligand), GPER modulators can act oppositely.
In breast cancer, there is an increased expression of GPER-1, which is a marker of the disease and a strong predictor of poor prognosis *. Conversely, the absence of GPER expression is associated with an excellent long-term prognosis for ER+ and PgR+ primary breast cancer treated with tamoxifen *. Despite this, treatment of cells with GPER agonists results in tumor suppression in vitro.
GPER-1 is of particular importance in triple negative breast cancer. Activation of GPER by its agonist (G-1) inhibited in vitro growth of breast cancer TNBC cells *.
The identification of GPER modulators is challenging because the biological effect of GPER-targeting molecules may be specific to different cells and tissues. As a result of screening, several effective chemical molecules have been identified that can selectively inhibit GPER * in vitro, but all of them are not yet available. And few natural modulators of GPER-1 are known.
• Baicalein, a flavonoid from Baikal skullcap (Scutellaria baicalensis) root, appears to be the most effective GPER modulator known. It can bind both ER and GPER * receptors, whereas tamoxifen blocks the ER but stimulates the GPER. The human dosage of baicalein equivalent to studies in mice * is 125 mg/day.
A large number of international studies have been published on the benefits of natural hormone supplements in case of deficiency. However, in medical practice, the main focus is mainly on estrogens. This applies not only to official medicine, but also to many clinics of alternative or complementary medicine.
Supplementation of major hormones appears to be the simplest, direct and well-controlled manipulation for hormonal deregulation. While an excess of estrogen requires various complex strategies to reduce it, any hormone deficiency is easily compensated by using their natural supplements. However, this approach requires supervision by a qualified specialist.
There are several rules for hormonal correction.
1. Reception of exogenous hormones is possible only after their deficiency confirmed by the analysis. Surprisingly, even medical professionals do not always follow this rule.
Unfortunately, the hormonal analysis of sex hormones in tissue presents certain difficulties.
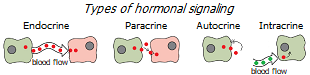
Firstly, it is easiest to find out the level of hormones circulating in the blood, i.e. involved in endocrine signaling. It is much more difficult to determine the level of hormones involved in paracrine and autocrine signaling. And even more so – in the intracrine, where the synthesis of active hormones from their precursors occurs inside the same cells on which they have their effect; therefore, there is no significant leakage into the blood vessels *. This means that the levels of intracellular concentration of sex steroids cannot be adequately assessed by measuring their level in the blood.
Meanwhile, intracrine production of estrogens in peripheral tissues in women is approximately 75% before menopause and almost 100% after menopause *. Therefore, the focus on serum levels in hormonal correction does not adequately reflect the level of hormones in the tissue, and can lead to a multiple overestimation of the dosage of supplements taken.
Secondly, to determine the biological activity of a particular steroid hormone, its level in the free (not associated with specific globulins or albumin) state should be determined. Unfortunately, the serum tests performed by most laboratories usually show the total levels of hormones present in the blood, ie. bound, and unbound (free) fractions together. It is believed that the ratio of bound and free hormones is in the range of 20:1-100:1, but its actual value is not exactly known.
The level of steroid hormones measured in saliva will probably more adequately reflect their level in breast tissue than the level measured in blood * *. But, unfortunately, saliva studies in clinics are very expensive, and the online laboratory service * * * is not available in all countries.
Third, estrogen and progesterone levels in reproductive women fluctuate depending on the day of the ovulation cycle *. Therefore, for an adequate assessment of the condition, at least two samplings of the material will have to be done – on the 11th and 20th day of the 28-day menstrual cycle, when, respectively, peaks in the concentration of estradiol and progesterone are observed *.
Hormonal analysis is carried out every 3 months, after which the specialist decides on the need for further therapy.
2. They take bioidentical, not synthetic, hormones. Molecules of natural or bioidentical hormones have a molecular structure that perfectly matches the structure of their receptor. Synthetic hormone molecules have a molecular structure that is different from what the receptor expects from them. For this reason, they cannot bind to their respective receptors properly and are unable to provide the same physiological activity as natural hormones. Even worse, many of them cause carcinogenic side effects * *.
Numerous studies show that progestins (synthetic analogs of progesterone) significantly increase mammographic breast density * and breast cell proliferation. This mainly happens through stimulation of the estrogen receptor * * and a significant increase in the number of genes expressed compared to the natural hormone (2'500 vs 600) *. At the same time, both natural progesterone and low-dose natural estradiol administered transdermally do not cause this negative effect * * *.
Women who take progestin plus estrogen as hormone replacement therapy (HRT) are twice as likely to have breast cancer as women who do not use HRT at all. And women who take natural progesterone in combination with estrogen instead of progestin, on the contrary, reduce this risk *. Oral contraceptives that contain synthetic forms of progesterone also increase the risk of cancer * *.
The only reasonable explanation for why synthetic hormones are still advertised and promoted instead of natural ones is the ability to patent their formula, and thanks to this, receive additional profit. Unfortunately, at the expense of women's health.
3. The dosage of exogenous hormones should be adequate. The dosage largely depends on the individual case, and is determined by a specialist on the basis of several indicators. Additives of hormones used not as a treatment, but as a compensation for their deficiency, should not create a concentration in the tissue higher than physiological. The levels of steroid hormones should not exceed the level that is observed in young years. Their ratio should also correspond to the ratio observed in younger years.
4. Supplements of a certain hormone should correct its ratio with the antagonist, and not cause an imbalance. To comply with this rule, you should check the level of all other major hormones, and not just the one of interest. That is, not only estradiol, progesterone, DHEA and testosterone should be checked, but also cortisol and thyroid hormones (TSH, T3, T4). The results of the analysis may show the need for correction of other hormones.
5. The method of administration of hormones must be effective. When administered orally, steroid hormones have poor bioavailability, and after entering the blood from the intestines, they undergo metabolic transformations in the liver, and 90% of them bind to proteins. In this form, they become water-soluble, easily distributed in the blood serum throughout the body, but at the same time lose their biological activity.
When administered through the skin, steroid hormones enter the bloodstream without first passing through the liver and, spreading through the lymphatic system, remain in a free, bioactive state. Thus, the local application of steroid hormones as part of the ointment will be more targeted, economical, and will allow better control of the amount of exogenous hormone that has entered the body.
Finally, correction of the level and ratio of hormones must be made at the appropriate time. So, in premenopausal women, the concentration of estradiol reaches a maximum in the luteal phase of the monthly cycle, and its excess should be fought, first of all, at this time. And melatonin is required by the body only at night, and it should be taken only at bedtime.
The table below shows the average serum concentrations of some hormones observed in women at various periods of life and the ovulation cycle.
The level and ratio of hormones are highly dependent on age, pregnancy, phase of the ovulation cycle, food composition and other circumstances.
Looking at the graph below of women's average sex hormone levels versus age, you can see that DHEA production begins to increase from 6-7 years of age, and as its concentration increases, testosterone, estrone and estradiol begin to be synthesized from it. At first, the growth of estrogens significantly overtakes the growth of androgens, promoting the growth and maturation of the reproductive organs and the mammary gland. When the formation of the specifics of the female body is completed, the concentration of androgens increases, which inhibit and balance the proliferative effect of estrogens.
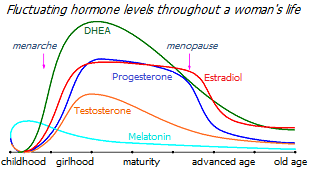
The decline in DHEA production, which begins after age 30 *, leads to a gradual decrease in its conversion not only into estrogen, but also into other hormones. In parallel with this, a gradual decrease in the production of progesterone and testosterone begins several years before menopause. The level of estrogen precursors in the serum is critically reduced, as a result of which there is first a reduction, and then a cessation of the production of estradiol by the ovaries. At the same time, an increasing amount of estrogen is synthesized from DHEA directly in tissues that are sensitive to estrogen.
That is, the drop in estradiol levels observed in menopause is only the finishing touch of previous functional changes, and not the center of the problem.
Moreover, if we assume that the decrease in estradiol is a protective reaction to an estrogen:androgen imbalance, then an attempt by exogenous supplements to restore its level without simultaneously restoring the estrogen:androgen ratio seems unreasonable.
In women with newly diagnosed breast cancer, a specific hormonal profile was noted, a feature of which is an increased level in saliva of estradiol (by 17.2%) and estrone (by 24.5%), and a reduced level of estriol (by 23%), testosterone ( by 15.5%) and sulfated dehydroepiandrosterone (DHEA-S) (by 17.5%) compared to control *. Other reports also reported significantly higher levels of total * or free * * estradiol in a group of women with newly diagnosed cancer compared to controls.
Thus, the balance between estrogen and androgen in them is significantly shifted towards hormones that promote proliferation and increase the risk of breast cancer.
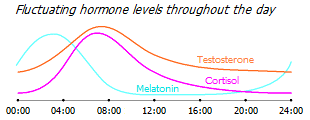
Cortisol levels are normally subject to cyclic diurnal fluctuations, rising to a maximum in the early morning and falling to a minimum in the late evening. In cancer patients, both an increase in the average daily level of cortisol in saliva and a smoothing of its daily rhythm are observed. But the more pronounced this deviation, the lower the number of natural killer cells, the more suppressed their condition, and the worse the prognosis for patients with metastatic breast cancer * *. These abnormal circadian patterns are more a cause of disease than a consequence of it, and may also serve to assess the risk of breast cancer in women with an undetected tumor *.
Testosterone levels are also highest in the first half of the day, and it is not surprising that the morning is the time of the most effective work. At the same time, melatonin levels are highest in the dead of night, but as cortisol levels rise, they begin to fall.
Progesterone (Pg) is an antagonist of estradiol (E2) and estrone (E1) *. Progesterone blocks estrogen receptors, resulting in tissue desensitization to estrogen * *. While estradiol promotes the expression of anti-apoptotic proteins (Bcl-2, survivin) and cell proliferation, progesterone promotes the expression of apoptotic proteins (p53) and counteracts proliferation *. The balance between these two hormones determines the fate of breast cells: death or growth. An increase in the concentration of estradiol increases the number of dividing cells, and an increase in the concentration of progesterone decreases *.
Although estradiol promotes and progesterone counteracts the development of cancer, estradiol is not a disaster and progesterone is a salvation. Estradiol is a hormone of femininity and youth, it exhibits a pronounced anabolic effect *, and the age-related drop in its level is associated with the onset of the decline of women's health and the development of age-related diseases *. In fact, the problem is not so much the level of estradiol itself, but the imbalance between estradiol and the androgens that restrain it, including progesterone. This means that estradiol, under the condition of its increased concentration, should not only be suppressed, but also compensated by the appropriate concentration of its natural antagonist, progesterone.
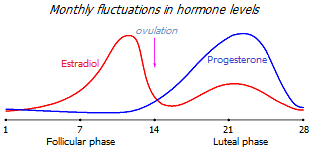
A mature woman experiences monthly cyclic changes in the balance of estrogen and progesterone, which genetically rearrange the behavior of hormone-sensitive cells in accordance with the requirements of the ovulation cycle. Depending on the period of the cycle, either the concentration of estradiol, which increases proliferation, or the concentration of progesterone, which works in the opposite direction, increases. As long as the concentration of both hormones is normal, these monthly swings keep the hormone-sensitive tissues in a healthy dynamic balance.
The ovaries of reproductive women produce up to 30 mg/day of progesterone during the peak of the luteal phase (around days 19-22 of a 28-day ovulation cycle). The proliferative phase of the ovulation cycle (the development of ovarian follicles) is over by this time, and an increase in progesterone production is required so that estradiol does not stimulate excessive tissue growth. Otherwise, in the long term (several monthly cycles in a row), excessive proliferation threatens with uncontrolled hyperplasia with the further development of uterine cancer. At the peak of the luteal phase, the level of progesterone in the blood serum of a healthy woman increases to an average of 18 ng/mL, i.e. more than 30 times compared to the follicular phase of the cycle (0.5 ng/mL) *, and 10 times less than during pregnancy (170 ng/mL) *. And in saliva, the level of free progesterone at the peak of the luteal phase rises to 75-270 pg/mL (1% of its level in serum) versus 10-25 pg/mL in the follicular phase, and versus 12-50 pg/mL in postmenopausal women *.
It follows that approximately 18 ng/mL of serum progesterone is required to contain estrogen-induced tissue proliferation; provided that the level of estradiol in the serum is within the normal range (160 pg/mL) *. It is easy to calculate that the serum ratio of progesterone:estradiol (Pg:E2) should be approximately 100:1.
During menopause and postmenopause, along with a decrease in the activity of the adrenal glands, the level of not only estrogen, but also androgen falls. Moreover, the concentration of progesterone in the tissue falls even earlier and more significantly than the concentration of estradiol. A chronic hormonal imbalance is growing, which is called «estrogen dominance». As estrogen inhibition is weakened, its proliferative effect is enhanced, increasing the risk of benign * and malignant * breast tumors.
Progesterone supplements in this case help not only reduce the risk of tumors, but also alleviate symptoms of estrogen over androgen dominance, such as decreased sex drive and interest in life, increased blood sugar, fatigue, cyclic migraine, fuzzy thinking, irritability, water retention, bone loss, obesity, thyroid dysfunction *. Moreover, taking physiological levels of natural progesterone seems to be quite safe. Several large-scale studies have not found an association of higher serum progesterone levels with the risk of breast cancer in both premenopausal * * and postmenopausal *.
In contrast to progesterone, both postmenopausal and premenopausal estradiol is associated with an increased risk of ER+/PR+ cancer by 1.9-2.6 times, estrone by 1.8-2 times, testosterone by 1.4-2 times, androstenedione by 1.5-1.7 times, DHEA – 1.1-2 times when comparing groups with the highest and lowest levels of each hormone * * * *. The last three hormones are precursors of estrogen, and when aromatase activity is high, their high concentration suggests high concentrations of estradiol and estrone, which increase the risk of cancer. However, androgens themselves may, on the contrary, reduce this risk *. Summarizing these facts, we still return to the fundamental reason for the risk of hormone-sensitive cancer – the dominance of estradiol over other hormones.
Adequate progesterone levels are important not only in postmenopausal women. In premenopausal women, high levels of endogenous progesterone, and with it a higher serum progesterone:estradiol ratio, are associated with a lower risk of invasive breast cancer *. If healthy premenopausal women have an average serum progesterone level on the morning of day 21 of the cycle is 17.6 ng/mL, then in women with hyperplasia it is 13.9 ng/mL, and in women with cancer it is 11.4 ng/mL *. Premenopausal women with regular menstrual cycles, who had the highest serum progesterone levels (> 13.5 ng/mL), versus women with the lowest levels (< 9 ng/mL), had an 8-fold lower risk of developing breast cancer *. Conversely, progesterone-deficient women, compared to women with normal progesterone levels, were 5.4 times more likely to have breast cancer and 10 times more likely to die from cancer *.
Women with the highest blood levels of progesterone during pregnancy, versus women with the lowest levels, showed a strong trend towards a lower risk of future breast cancer *. For example, for women under the age of 51, the difference in breast cancer risk between the two groups was as high as 70%. These data are incomplete, but they give a target that we should aim for – about 18 ng/mL of progesterone in serum during the peak of the luteal phase at the corresponding concentration of estradiol.
In experiments in vitro, progesterone at a concentration close to that in the third trimester of pregnancy showed a strong antiproliferative effect in ER+/PR+ tumor cells *. Unlike chemotherapy drugs that act on all cells equally, progesterone stimulates the death of only those cells in which the normal regulation of the suicide program (i.e., cancer cells) is disrupted. And in experiments on animals, progesterone enhanced the antiproliferative effect of tamoxifen *. Thus, progesterone supplements could be beneficial for progesterone deficient women. Indeed, progesterone gel applied to the breast inhibits estrogen-induced cell proliferation * and can halve the risk of breast cancer in premenopausal women with benign tumors *.
The concentration of progesterone in the saliva of healthy premenopausal women during the luteal phase is usually in the range of 0.3-0.5 ng/mL. The physiological dosage of transdermal progesterone supplementation to achieve an average salivary level of 0.5 ng/mL is 12-15 mg/day *. To do this, you can use both prescription and homemade cream, which is prepared by mixing 900-1'000 mg of natural progesterone with 60 grams of neutral cream. The cream is applied directly to the breast (1 g/day, i.e. about a quarter of a teaspoon). Increasing the dosage to this rate should take place smoothly, within 2 weeks or more.
Progesterone (15 mg) is rapidly absorbed after application to the skin, and peak levels in saliva reach 12 ng/mL after 2-3 hours. After 8 hours, its level drops by 80% – to 1.5-3 ng/mL, and after 24 hours – to 0.3 ng/mL *. If the task is to avoid sharp hormonal surges, then the procedure can be divided into 2-3 doses, taking a correspondingly smaller portion of the cream.
Transdermal delivery of steroid hormones is the preferred form of delivery for female breast treatment. Studies on local delivery of sex hormones to various body environments suggest that in this case they will be delivered to tissues predominantly not via the bloodstream, but rather via the lymphatic system. It follows that local hormonal therapy will be more beneficial for tissues with a well-developed lymphatic network, such as the mammary gland, uterus, brain and immune system. While tissues with a less developed lymphatic network (bones and skeletal muscles) will be less affected by hormonal therapy. On the other hand, sex hormones (such as E2 and Pg) delivered locally will be less effective in stimulating bone growth in menopausal osteoporosis, and testosterone will be less effective in increasing muscle mass in androgen deficiency-induced sarcopenia *.
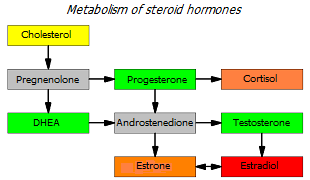
Progesterone is the precursor hormone for estrogen. From it, androstenedione and testosterone are first synthesized, and already them with the help of the aromatase enzyme – estrone and estradiol. This means that with high aromatase activity (which happens with age), progesterone supplements can cause a progesterone:estradiol imbalance. This implies the conclusion that for successful hormonal correction it is worth using at least weak (natural) aromatase inhibitors, such as pomegranate juice, licorice, ginkgo biloba, melatonin, mushroom powder.
Again, we emphasize that not only the level of the hormone itself is important, but also its relationship with the levels of other hormones, as well as the cyclical change in these levels. Premenopausal women with estrogen deficiency usually do not receive progesterone. Premenopausal women with excess estrogen start taking progesterone on day 12 of the cycle, and stop taking it 2 days before the planned end of the cycle. And postmenopausal women take it 3 weeks a month continuously, and then a week off *. If the hormonal imbalance of progesterone:estradiol in favor of estradiol is insignificant, the dosage of the cream should be less than 1 g/day. And if the cream is used as a 2-week preoperative preparation, the dosage can be doubled; and it will be better if it falls on the luteal phase of the cycle. In any case, regular monitoring of the level of both hormones is required.
Extremely high levels of progesterone (about 186 ng/mL in the luteal phase) are also associated with an increased risk of breast cancer, and are just as dangerous as its deficiency *. Progesterone enhances the expression of an immunomodulatory protein known as progesterone-induced blocking factor (PIBF) *. This protein is produced in excess during pregnancy, preventing immune killer cells from destroying the fetus. Thus, an excessive amount of progesterone will suppress the activity of immune NK cells, promoting tumor growth. This once again emphasizes the need for constant laboratory monitoring of an adequate level and balance of the main sex hormones.
A small number of plants with progestogenic activity are known, which, however, is low and very poorly studied *.
• Red clover (Trifolium pratense) and its ethanolic extract have been reported to exhibit a weak progestogenic effect *. Apparently, this is due to the fact that kaempferol present in it is a partial agonist of PR.
• Vitex (Vitex agnus-castus) contains small amounts of apigenin, which appears to have a progestogenic effect *, confirmed in experiments on monkeys * *.
Special studies show that natural progesterone, in contrast to its synthetic forms (progestins), does not contribute to breast cancer, but rather protects against it *. And it appears to inhibit the invasion and migration of breast cancer cells, regardless of their progesterone receptor status *. In addition, progesterone may also be helpful in the prevention of cardiovascular disease and neurodegenerative conditions such as stroke.
The combination of estrogen with progesterone in HRT carries a lower risk of breast cancer than estrogen alone; however, in any case, long-term HRT (more than six years) may carry this risk *.
Before attempting to compensate for a progesterone deficiency through supplementation, it is wise to try to normalize the hormonal balance through natural means, such as reducing exposure to toxins and stress; moderate physical activity; quality sleep; healthy diet (high intake of carbohydrates and fiber, low intake of fats and animal proteins). The emotional state is important. As stress increases, cortisol levels rise, and since cortisol is synthesized from progesterone, chronic stress depletes progesterone *. Relaxation is recommended to reduce the negative effects of chronic stress.
Testosterone (Т) is the main androgen not only in men, but also in women, although women have significantly lower testosterone levels. Just like estradiol and progesterone, testosterone in women is produced in the ovary, and provides a balance between estrogen and androgen *. The addition of testosterone to estrogen markedly decreases the ERα:ERβ ratio *; and because ERα activates a number of genes associated with breast epithelial proliferation, it reduces the risk of breast cancer *.
Super-high androgen levels in healthy women inhibit breast proliferation so strongly that it can cause breast atrophy, which is seen in female athletes * and transsexuals * taking high doses of androgen supplements. Androgen excess also causes polycystic ovary syndrome, despite the fact that the relative risk of breast cancer can be halved * *. Conversely, androgen suppression in men is associated with gynecomastia *, and mutations in the androgen receptor cause breast cancer in men *. It has also been found that the combination of an antiestrogen (tamoxifen) with an androgen (fluoxymesterone) increases the effectiveness of the antiestrogen and inhibits the development of the disease compared to the antiestrogen alone * *. These observations suggest a protective role for bioavailable androgens in counteracting the proliferative effects of estrogens on breast tissue * *.
A severe drop in testosterone levels begins in women as early as 30 years of age * and increases towards menopause, which is associated with an age-related increase in the risk of breast cancer. At 30 years of age, there is an increase in the incidence of hormone-sensitive breast cancer, while the increase in the incidence of other types of cancer occurs mainly at the age of 50.
In the saliva of postmenopausal women with breast cancer, compared with healthy women of the same age, both the level of DHEA-S and the level of free testosterone are significantly lower (by 17.2% and 15.5%, respectively) *. Thus, the balance of estrogen:androgen in them is biased in favor of estrogen. With modern treatment, the symptoms of menopause and ovarian failure are usually corrected by supplementation with only estradiol and progesterone, without taking into account testosterone, which does not provide an adequate balance of estrogen and androgen.
Testosterone deficiency is a problem, for the most part, of postmenopausal women. However, some other women may experience an increased need for exogenous testosterone. For example, women taking a high dose of metformin (1'500 mg/day) *. Metformin reduces the level of estradiol, however, it does this mainly by reducing the level of its predecessor, testosterone *.
There is a natural fear that testosterone can act counterproductively in breast cancer, since it will be converted to estradiol with the help of aromatase. But that risk seems to depend on menopausal status. In premenopausal women, elevated testosterone levels are not associated with an increased risk of breast cancer. But in postmenopausal women who suffer from estrogen depletion and have increased adipose aromatase activity, higher testosterone levels are associated with a higher risk of breast cancer *.
As if to prove it, a survey of postmenopausal women found a 248% increase in incidence in those who used the combination of estradiol with testosterone, and a 15% increase in incidence in those who used estradiol alone, compared with those who did not use hormone replacement therapy at all *.
n contrast to this observation, a manipulative study in premenopausal primates showed that testosterone reduced estradiol-induced proliferation by 40% while progesterone failed to do so *. Conversely, blockade of androgen receptors in normal female primates led to an increase in the proliferation of the mammary gland epithelium by more than 2 times. Moreover, the addition of small physiological doses of testosterone to standard estrogen therapy almost completely attenuated estrogen-induced proliferation *. Researchers have drawn attention to the fact that for hormone replacement therapy to be successful, all three of these hormones must be used simultaneously, but not one or two of them.
A manipulation study involving postmenopausal women showed similar results. Whereas estrogen/progestin hormone replacement therapy significantly increased mammary epithelial proliferation, testosterone supplementation reversed this effect *.
Several large-scale studies have been conducted to elucidate the effect of serum concentrations of various sex hormones on the risk of breast cancer. In a comparison of five groups of postmenopausal women with different serum hormone concentrations, there was a significant increase in breast cancer risk between the highest and lowest concentration groups at higher absolute values of androgens (DHEA-S, androstenedione, testosterone) and estrogens (estrone, estradiol); and especially free estradiol (by 2.3 times) and free testosterone (by 2.5 times) * * * *. The researchers agreed that, at the tissue level, breast tumor development may be due more to excess estrogen than androgen. But in contrast to this agreement, their conclusion was categorical that "our data provide strong evidence against the use of androgens in postmenopausal hormone therapy."
Meanwhile, breast tissue has aromatase activity *, converting testosterone to estradiol in situ. Therefore, the association noted in the above studies between high testosterone levels and cancer risk may rather reflect an increase in estrogen levels with an increase in androgen levels, i.e. reflect aromatase activity. Where estrogen was taken into account, no association was found between testosterone and breast cancer *.
Unfortunately, all this huge work has been focused on comparing the absolute values of hormones in serum, ignoring the comparison of the ratio of their values in the place that interests us – in the tissue. However, even serum values can tell a lot about the risk of disease in postmenopausal women. In studies where the data were open-ended and allowed comparison of absolute values, in the «case» groups compared to the «control» groups, an average increase of 7% in the ratio of estradiol:testosterone in serum * * * * * was observed.
A similar extensive work has been done on premenopausal women. Here, progesterone and testosterone, unlike other hormones, had practically no effect on the incidence *. However, the hormone ratios were also not considered here. But the study of open data works on this topic also give an average of 7% preponderance of the ratio of estradiol:testosterone in serum in the «case» group against the «control» group * * * *.
The multinational study also showed that in addition to DHEA-S and testosterone, other sex hormones showed a significant linear association with breast cancer in postmenopausal women. Here, women diagnosed with breast cancer after an average of 3 years had an estradiol:testosterone ratio 17% higher than healthy control women *. The tissue estrogen:androgen ratio difference should therefore be even higher * *.
Other clinical studies have also not found a negative association of physiological doses of testosterone (up to 300 μg/day) with the risk of cancer in postmenopausal women * *. In a 6-month clinical study, in a group of postmenopausal women who used transdermal testosterone (300 μg/day) in addition to a combination of estradiol (2 mg/day) and synthetic progesterone (1 mg/day), breast cell proliferation almost completely stopped, in while in the group without testosterone supplementation, cell growth increased 5 times from baseline *. Postmenopausal women taking a testosterone supplement (300 μg/day transdermally) also experience a significant improvement in their quality of life in terms of psychological well-being and sex life *.
A systematic review of publications has shown that transdermal testosterone use in postmenopausal women does not increase the incidence of breast cancer *. For the final dispelling of fears, you can combine testosterone with aromatase inhibitors. The best way to deliver testosterone will probably be the implantation of capsules with hormonal contents. This will prevent it from reaching other parts of the body through the general circulation causing unwanted side effects, and will also avoid binding of free testosterone by the liver.
Performed every 3 months, subcutaneous breast implantation of 2 capsules with a combination of testosterone (120 mg) and an aromatase inhibitor (anastrazole, 4-8 mg) significantly reduces and even reverses the growth of ER+/PR+/AR+ breast tumors *. Three implants (60 mg of testosterone and 4 mg of anastrozole) for 46 days of therapy reduced the volume of the ER+/PR+/AR+ tumor in the patient by 7 times, and in 13 weeks by 12 times *. A similar regression of the tumor within 4 months was observed in the patient due to the implantation of testosterone with anastrozole, another aromatase inhibitor *. In older women who took this therapy as a prophylaxis, the incidence was more than 3.5 times lower than expected *. The estimated dosage of testosterone produced by the capsule for 100 days was about 20 μg/kg, or 1'500 μg/day. Because of this, the serum testosterone level in the patients was several times higher than the physiological level, and even more so than the initial level *.
Success has also been reported in the treatment of testosterone alone in advanced metastatic breast cancer that has become resistant to hormone therapy. Here, the dosage was significantly lower: patients received intramuscularly testosterone propionate (250 mg twice every four weeks, and then regularly once every four weeks). In 41.5% of patients, the disease continued to develop, in 41.5% it stabilized, in 15% there was a partial regression, and in 2% a complete regression *. Survival of patients on average was increased by 1 year. However, the problem with testosterone is the same as with other hormones – cells can develop insensitivity to it, thereby reducing the effectiveness of hormone therapy over time.
As with any other supplement, in order to determine the need for testosterone supplements and to determine their dosage, it is necessary to know its current blood level. For women of reproductive age who are not deficient in testosterone, or women who have contraindications, testosterone supplementation is not recommended *. In addition, the hormone receptor profile of the tumor should be known because many breast cancers express the androgen receptor *, including those that do not have estrogen or progesterone receptors (TNBC tumors) * *. For patients with these tumors, treatment with androgen receptor inhibitors rather than testosterone treatment may be considered.
Testosterone production in a reproductive woman is 300 μg/day. Thus, in confirmed moderate testosterone deficiency in postmenopausal women, a dosage of 150 μg/day may be sufficient to eliminate it. In this case, it would be useful to combine testosterone intake with natural aromatase inhibitors present in foods. For overweight postmenopausal women, metformin (500 mg/day) may be taken as an inhibitor.
Testosterone, just like estradiol, estrone, and progesterone, is best used topically, not by mouth *. Transdermal delivery ensures high bioavailability of free testosterone. Sublingually, testosterone is approximately 99% absorbed, in the same way as topically applied testosterone in dimethyl sulfoxide solution; while only 20% of testosterone is absorbed from transdermal cream - the same as testosterone taken orally.
To prepare testosterone ointment, 10 mg of injectable testosterone enanthate (75% of actual testosterone) is mixed with 30 g of castor oil and 25 ml of 99% dimethyl sulfoxide *. 1 g is applied in the morning 5 days a week – on the chest for problems with the mammary gland, or on the vagina to prevent vaginal atrophy. But of course, before you start using testosterone, just like any other sex hormone, you should make sure that you are deficient in free testosterone, and then adjust the dosage for its current level and body weight.
Dehydroepiandrosterone (DHEA), so-called «hormone of longevity», produced in the adrenal glands. It is a precursor hormone for estrogen and androgen; and about half of all androgen is produced in women from this hormone *. DHEA exhibits an antitumor effect * *, in particular by inhibiting the proliferation and migration of cells *, including breast cells * * *, as evidenced by numerous preclinical and observational studies *. Conversely, lower DHEA levels are associated with some types of cancer, including breast cancer *.
The advantage of DHEA over other androgens is that, at physiological doses, it is converted to androgens and/or estrogens only in certain intracrine target tissues that have the appropriate physiological enzymatic mechanism. Due to this, the action of DHEA is limited only to these tissues, leaving other tissues unchanged. And thus minimizing the potential side effects seen in premenopausal women with systemic administration of androgens or estrogens *.
As the body ages, the production of estrogen and androgen in peripheral target cells decreases due to a decrease in adrenal synthesis of DHEA and DHEA-sulfate (DHEA-S). During menopause, this decline reaches an average of 70% *, and continues further up to 95%, although with a wide variation in indicators in different women *. If a woman produces 8-16 mg/day of DHEA in the reproductive age, 6-8 mg/day in premenopause, and 2-5 mg/day and even lower in postmenopause *. The reasons for this are still unknown. To compensate for the lack of estrogen, tissues increase the local expression of aromatase, which allows them to synthesize the missing estrogen from androgen *.
The problem with DHEA is that, unlike other hormones, there is no internal feedback that regulates its levels. At the very least, no intrinsic mechanism is known to stimulate an increase in DHEA production when levels drop *. This means that in case of DHEA deficiency, only exogenous supplements can bring its level to the required level.
Many of the main symptoms of menopause can be rapidly relieved with topical DHEA without the risk associated with estrogen exposure *. The advantage of DHEA over other androgenic hormones is that it is converted to other hormones only in tissues with the appropriate enzymatic mechanism, without negative side effects on other tissues. That is, DHEA supplements may be safer than progesterone supplements, and even more testosterone supplements, which show an effect in all tissues that have an androgen receptor. For example, oral administration of 50mg/day DHEA for 6 months did not produce any negative changes in endometrial thickness in postmenopausal women * due to the absence of aromatase, which converts DHEA into estrogens * *. Topical application of DHEA 6.5 mg/day failed to reverse endometrial atrophy in postmenopausal women *, but improved other menopausal symptoms *.
Several experimental studies have found that increased levels of endogenous DHEA-S do not increase the risk of breast cancer, unlike other sex hormones. Some have even shown that premenopausal levels of DHEA or its sulfate are inversely related to breast cancer risk * *. On the other hand, it has been reported that when DHEA production in different parts of the body is extremely low, it may indicate the presence of a malignant tumor somewhere in the body *.
Sometimes DHEA is recommended for people over 60 as an anti-aging agent (50 mg/day in the morning) *. Oral DHEA 100 mg/day has shown high safety but poor activity in metastatic breast cancer in clinical trials *. However, there are publications that consider dosages up to 12.5 mg/day to be optimal, and long-term dosages of more than 25 mg/day are considered dangerously high *.
Over the years, the synthesis of sex hormones in humans moves from the endocrine glands to peripheral tissues *. And after menopause, when ovarian production of estradiol ceases, estrogens are produced almost entirely locally from DHEA and DHEA-S derived from the adrenal glands *. Administration of 4×400mg DHEA orally for a month has been shown to increase both serum estrogen and androgen levels in postmenopausal women *.
DHEA can be administered once a day *. DHEA supplementation also significantly reduced levels of pro-inflammatory molecules (TNF-α and IL-6) in older adults *, serum cholesterol levels in postmenopausal women *, reduced abdominal fat mass, and improved insulin tolerance *. When administered orally in women, the concentration of testosterone, estrone and estradiol increases, and when administered transdermally, mainly estrone and estradiol *.
Interestingly, of all the other sex hormones, DHEA-S increased the risk of postmenopausal breast cancer the least at a doubling of its blood levels *. This makes DHEA a promising candidate for hormonal management in postmenopausal women, instead of a combination of exogenous estradiol and testosterone. Although it may require a combination of low doses with aromatase inhibitors.
Thyroid hormones.
Higher serum total T4 concentrations are associated with both premenopausal and postmenopausal breast cancer, but the association is twice as strong in premenopausal women. At the same time, in premenopausal women, the detrimental effect of T4 decreases as body mass index rises, while in postmenopausal women, the opposite pattern is observed.
Lower concentrations of T3 are also associated with breast cancer, but the protective role of T3 is more pronounced in premenopause than in postmenopause. No significant associations were found with any other thyroid function parameters (TSH, Tg, Tg Ab, or TPO Ab) *.
To support thyroid health, the following supplements may be helpful in many cases.
• Iodine deficiency, which occurs in almost all European countries *, leads to thyroid dysfunction. Iodine is the main element of the thyroid gland; it is essential for the synthesis of thyroid hormones, and also helps regulate levels of the stress hormone cortisol * and contributes to the normal immune function of leukocytes and phagocytes *. Dosage: 5 mg/day. Seafood is rich in organic iodine. Among plant products, the record holder for iodine content is the Feijoa fruit (Feijoa sellowiana).
• Selenium is component of the enzyme that converts T4 to T3. Inflammation, as well as high levels of oxidants, reduces the amount of selenium in the thyroid gland. Dosage: 600 μg/day. The richest source of organic selenium is Brazil nuts; followed by seafood.
• Zinc * *, copper and iron *. Deficiency and/or imbalance of these metals can cause ineffective regulation of thyroid hormones. Zinc dosage: 25 mg/day. Adequate consumption of vegetables can largely eliminate their deficiency, and dietary diversity can eliminate their imbalance.
• Vitamin D3. Widespread vitamin D deficiency increases the risk of autoimmune thyroid disease *. Dosage: 2'000-4'000 IU/day.
• Vitamin Е. May help reduce oxidative stress associated with underactive thyroid *. Dosage: 15 mg/day.
• Vitamin А. Deficiency of retinoic acids causes thyroid dysfunction *. Dosage: 1 mg/day.
• Vitamin В12 deficiency is accompanied by thyroid diseases *. Dosage: 3 μg/day.
• Dehydroepiandrosterone (DHEA) and pregnenolone. Usually both of these hormones are deficient in patients with thyroid insufficiency *.
• Natural sources that improve thyroid function and thyroid hormone levels: turmeric (1g/kg of food) and other antioxidants *; myrrh gum (equiv. 1'250 mg/day) *, golden root *; ginseng (500 mg/day extract) * * and ashwagandha (500 mg/day extract) *. In patients with subclinical hypothyroidism, ashwagandha root extract (600 mg/day) significantly improved serum TSH, T3, and T4 levels compared to controls * for 8 weeks.
Serotonin regulators. Serotonin (5-HT) regulates epithelial homeostasis in the mammary gland through a number of receptors. Dysregulation of serotonin signaling negatively affects breast tumors by enhancing cell proliferation and increasing resistance to apoptosis *.
• Black cohosh (Actaea racemosa, Cimicifugae racemosae) at low to moderate doses does not exhibit either competitive binding to estrogen receptors or regulation of estrogen-inducible genes *. An overwhelming number of clinical studies report no change or a slight decrease in serum estradiol levels in connection with the intake of cohosh *.
Taking black cohosh rhizome extract can markedly alleviate the psycho-vegetative symptoms of menopause such as hot flashes * * in a significant number of women and reduce the risk of relapse in patients taking tamoxifen *. Some studies have also found beneficial effects of black cohosh on bone tissue * *. The isopropanol extract of black cohosh in vitro inhibits proliferation rather than stimulates it *, and the ethanol extract inhibits aromatase activity in breast cancer cells (MCF-7) *. Black cohosh has a strong preventive effect in women against breast cancer *, and after anti-cancer therapy, it triples the duration of the disease-free period before recurrence *.
Unlike tamoxifen, cohosh does not have negative estrogenic effects in either the uterus or the mammary gland * * *, and does not adversely affect the liver *. The amount of accumulated data indicates that the action of cohosh occurs due to hormonal regulation through the brain; in particular by controlling serotonin * as well as dopamine, norepinephrine and gamma-aminobutyric acid. The effect of the action does not begin to appear immediately, but after a few weeks of admission. Dosage: 40 mg/day of dry herbal raw materials, which is equivalent to 4-8 mg of dry extract *, but no more.
However, cohosh remains one of the most controversial drugs used in breast cancer, and the safety of its use is still not completely clear.
• Valerian (Valeriana officinalis) is widely used to help you fall asleep and improve the quality of your sleep. Although the active ingredients of valerian have not been identified, valerian root extract has been reported to affect dopamine * and serotonin * mechanisms and may be effective in alleviating menopausal symptoms in women, especially reducing the frequency of hot flashes and night sweats *. The dosage of dried valerian root in clinical studies was 765 mg/day *, and showed no negative side effects.
Stress hormones regulators. The body responds to stress with an immediate release of adrenaline and norepinephrine, which increases heart rate, blood pressure and sharpens attention, allowing you to better respond to a threat to life. However, chronic stress keeps these hormones abnormally high even in the absence of danger. The activation of the corresponding cell receptors by hormones causes the cells to lose their ability to inhibit division and thus increases the risk of cancer *.
Stress stimulates the adrenal glands to release large amounts of cortisol. Cortisol can interfere with the production of other hormones, such as prolactin and estrogen. Prolactin can cause pain in the mammary glands, and enough has been said about the dangers of excess estrogen.
Beta-blockers are a class of drugs that reduce the effects of adrenaline and norepinephrine by blocking adrenal receptors. Diabetics taking beta-blockers have a 67% lower risk of developing cancer than diabetics not taking them. Just one month of taking beta-blockers reduces this by 13% *. Patients taking beta-blockers can reduce their risk of dying from breast cancer by 50% compared to patients not taking them *. In addition, women who were already using beta-blockers at the time of breast cancer diagnosis had a 56% higher overall chance of survival *.
Patients with HER2– breast cancer taking beta-blockers significantly increased progression-free survival *. However, the dosages used in the reviewed studies varied over a wide range, making it impossible to accurately determine the recommended dose. Therefore, you can build on the values recommended for other diseases. For propranolol, this can be 80-160 mg/day, and for carvedilol – 12.5-25 mg/day.
Unfortunately, beta-blockers can lower blood pressure and also have a number of other side effects, including being suspected of increasing the incidence of myocardial infarction and stroke *. Decreased survival rates have also been reported in patients taking beta-blockers *. Thus, the need for the use and dosage of beta-blockers must necessarily be determined solely by the attending physician.
The best known stress hormone is cortisol. Thanks to cortisol, the body’s strength and resources are rushed to solve a critical «fight or flight» situation. Blood pressure, blood glucose levels, the ability of blood to form blood clots increase, and the concentration of lipids in the blood increases. In this case, the improvement in blood supply to the muscles and brain occurs due to a deterioration in the blood supply to other organs. In addition, it weakens the immune defense. Thus, chronically high levels of cortisol, like other stress hormones, are harmful. Fortunately, as the critical situation resolves, cortisol levels usually return to baseline.
Under normal conditions, cortisol levels are subject to diurnal fluctuations (rise in the first half of the day and decline in the second), but there are various violations of this cycle. In particular, due to chronic stress, the concentration of cortisol in the blood can remain at a high level for too long. There are no effective natural remedies that can regulate cortisol levels. However, the following measures can be used for this purpose:
- moderate but not strenuous exercise in the morning (see «Healthy Exercise» section †)
- improved sleep quality (see «Healthy Sleep» section †);
- avoiding, if possible, sources of stress (toxic people, negative information, etc.);
- pleasant atmosphere – work for pleasure, hobbies, communication in a good company, pleasant music, dancing, entertainment, a pet, etc.);
- creation of psychologically healthy relationships with others (family, colleagues, friends);
- walks in nature, watching comedies, prayer or meditation.
Other hormonal regulators.
• Melatonin is a powerful antioxidant and neuroprotective hormone that promotes deep, quality sleep. It also regulates the balance of estrogen and insulin-like growth factor IGF-1, inhibits aromatase activity, counteracts the activity of estradiol. Melatonin is an over-the-counter remedy. Dosage: 3-20 mg sublingually at bedtime. Long-term use of more than 9 mg/day is not recommended without a doctor's prescription.
• Thymoquinone is the main component of the seeds of Black cumin (Nigella sativa), does not affect the production of hormones, but allows you to retain free testosterone in the serum. Extra virgin seed oil, rich in thymoquinone, apparently due to its antioxidant properties, also contributes to lowering systolic and diastolic blood pressure, as well as lowering fasting blood sugar, triglycerides and HDL-cholesterol, uric acid and C-reactive protein *. Dosage: 1-3 g/day of oil or 3-5 g of seeds *.
• Beta-sitosterol is a phytosterol that prevents the conversion of testosterone to dihydrotestosterone by blocking the corresponding enzyme (5α-reductase). For men, it may be more beneficial, but for women, it also helps keep testosterone from being metabolized into other hormones. The modern diet provides approximately 80 mg/day of phytosterols, while the Japanese or vegetarian diet provides over 340 mg/day *. Rich plant sources of beta-sitosterol and other phytosterols are cereal germ, sesame, pistachios, almonds, macadamia, sunflower seeds *.
• Peony (Paeonia lactiflora). The root of the plant allows you to reduce the level of another potentially carcinogenic hormone – prolactin *. Dosage: 15 ml (1 tbsp) of root ethyl extract per day *.
• Thioridazine is an antipsychotic antipsychotic that selectively targets neoplastic cells and antagonizes dopamine receptors (DR), which are expressed in both normal breast cancer cells and cancer stem cells *. In patients with schizophrenia treated with DR antagonists, there was a reduction in the incidence of rectal, colon, and prostate cancer *. Dosage: 4×25 mg.
• 8-prenylnaringenin (a component of hops) reduces the level of follicle-stimulating and luteonizing hormones *.
Hormonal influence on women's health remains one of the most powerful, but at the same time the most subtle tool, and requires a responsible and professional approach.