Health Strategy.
Providing the body with the building and energy materials it needs – macro- and microelements, occurs through food and drink.
Macronutrients are required by the body to build tissues, maintain a constant osmotic pressure, ionic and acid-base balance. Micro- and ultramicroelements are involved in the construction and operation of enzymes, hormones, vitamins and biologically active substances. A slight deficiency or excess of chemical elements may not be noticed until this anomaly becomes too obvious.
For the body, the difference between insufficient and deficiency of micronutrients is only in the degree of health disorder. A insufficient of micronutrients may go unnoticed for a long time, but at the same time secretly contribute to the emergence of many painful conditions of an inexplicable nature. Micronutrient deficiency sooner or later leads to obvious dysfunction of cells and organs.
Micronutrient deficiencies can lead to cell genetic damage *. Lack of vitamins В12, В6, C and E; folic acid; niacin and zinc in their ability to oxidative damage to DNA can be compared to exposure to radiation *. Supplements of missing elements can reduce the risk of morbidity, enhance the therapeutic effect of the main treatment, and ensure the safe metabolism of estrogen. As well as prevent other degenerative diseases and improve overall health.
Chronic imbalance or deficiency in the body of any macro- and microelements necessary for each human tissue and organ, ultimately leads to an imbalance of physiological processes and dysfunctions of the body, manifested in the form of various diseases. This makes you take seriously the preparation of your diet, and its adjustment due to the addition of those substances that the body lacks.
A significant part of the supplements discussed below are essential substances that are deficient for the majority of the population in many countries of the world. They are called indispensable because the human body is not able to synthesize them on its own from other molecules, and therefore is forced to receive them from outside. The ideal source of providing the body with a sufficient amount of all the necessary essential substances is a reasonable amount of a balanced diet. Unfortunately, very often they enter the body in quantities insufficient for normal metabolism. In such cases, nutritional correction in the form of supplements is required, and therefore such supplements are called corrective.
Some of the essentials may be deficient for geographic reasons, such as iodine, selenium or vitamin D. Others may be due to a metabolic disorder or due to a bacterial imbalance in the gut. Still others – due to an unbalanced diet. As a result, there is a concern that the restriction of the daily caloric intake of 2'200 kilocalories that we have planned may not provide an adequate intake of necessary, including irreplaceable substances.
Indeed, an analysis of the four most popular scientifically developed nutritional systems in the United States * showed the reality of such a threat. All these diets turned out to be unbalanced, and failed to provide a sufficient level of dietary intake of vitamins В4, В5, В7, E, D, zinc, iodine and chromium. In order to meet the recommended intake of vitamins and minerals, the foods included in these diets will have to be consumed in quantities that will provide 3'500 kcal/day. That almost doubles the 2'000 kcal/day recommended by the American Institute of Nutrition.
Increasing food intake does not automatically improve nutritional status unless dietary patterns are adjusted. According to the US Center for Disease Control and Prevention 2012 *, a significant portion of the US population is deficient in certain vitamins and minerals while consuming an excess of calories.
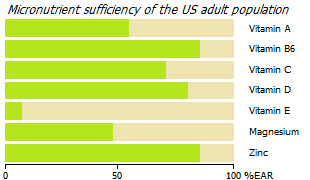
According to the US National Health and Nutrition Study (NHANES) 2005, 93% of US adults do not meet the estimated average requirement (EAR) for vitamin E, 56% for magnesium, 44% for vitamin A, 31% for vitamin C, 14% in vitamin B6 and 12% in zinc *. In addition, vitamin D deficiency is a worldwide problem. It is estimated that 1 billion people in the world are either deficient or insufficiency in vitamin D *.
An analysis of nutrition † of groups of countries with different incidence of breast cancer shows that even in «prosperous» countries, insufficient intake of certain vitamins (A, β-carotene, B2, B9, D, E) and chemical elements (calcium, iodine, fluorine) with food is a widespread problem. And if the nutritional deficiency of vitamin D can be compensated by solar radiation, then with other substances the problem can be solved only by their additional consumption.
The need for supplementation may increase with certain medical conditions, as well as with many popular modern diets. An attempt to reduce the calorie content of a diet by reducing its volume can also lead to a reduction in the supply of essential substances to the body, which forces more careful attention to the compilation of the nomenclature of the diet. Although food is a more beneficial source of minerals and vitamins than supplements, it is challenging to fully meet dietary micronutrient requirements.
Indeed, compiling a complete diet with a calorie content of already 2'500 kcal, and even more so with a calorie content of 1'600 kcal, is not an easy task. And even with a perfectly composed diet, you have to strictly monitor it in order to avoid a lack or imbalance of micronutrients, which is also quite difficult, troublesome and uncomfortable.
In addition, in some cases there may be individual genetic characteristics that force the use of one or another supplement. It should be noted that if the deficiency of one or another element or essential substance is the result of genetic problems or some kind of disease (diabetes, acidosis, chronic systemic inflammation, etc.), then their supplements will support the body, but will not fundamentally solve the problem.
Finally, there is a problem called the habitat crisis. Our food year by year becomes poorer in minerals and trace elements *, which is associated with the depletion of fertile soil due to the intensification of agriculture. A modern person does not receive such an amount of micronutrients that a person received some two or three hundred years ago, with the same volumes or with the same calorie content of food. All this also inclines to the need to compensate for the shortcomings of food consumption by taking certain supplements.
A variety of single nucleotide polymorphisms (SNPs) can lead to a critical deficiency in the body of certain metabolically important agents. The most common negative anomalies and/or their combinations are:
- poor absorption of vitamin В12 – polymorphisms rs602662 (A;G), (G;G), rs601338 (A;G), (G;G),
- poor metabolism of folic acid – polymorphisms rs1801133 (T;T),( C;T), rs1801131 (C;C), (A;C),
- poor synthesis of vitamin B – polymorphisms rs10741657 (G;G), rs12794714 (А;А), rs2060793 (А;А),
- poor synthesis of vitamin A – polymorphisms rs7501331 (С;Т) (Т;Т), rs12934922 (А;Т) (Т;Т),
- poor synthesis of phosphatidylcholine – polymorphisms rs7946 (Т;Т) (С;Т), rs174548 (G;G) (C;G),
- poor conversion of ALA to EPA – polymorphism rs1535 (G;G),
- impaired fatty acid metabolism – polymorphisms rs429358 (C;T), rs7412 (C;C), rs429358 (C;C), rs7412 (C;C).
They can be identified by genomic analysis. Such people should seriously consider taking appropriate supplements on a regular basis with a specialist.
According to the experience of the Keith Block Center for Integrative Cancer Treatment, the use of a complex of vitamins, minerals, trace elements in combination with a healthy diet and lifestyle can approximately double the survival rate of patients with advanced metastatic breast cancer compared to patients with conventional treatment alone *.
However, it remains unclear what kind of supplements a particular patient should take, in what doses, for how long, and whether he needs to take them at all. Despite typical regional deficiencies, each person may have specific individual deficiencies and/or imbalances of vitamins and minerals, which can only be determined with the help of tests, and even then not always with sufficient accuracy. Thus, the issue of taking supplements is decided strictly individually.
At the same time, one should beware of thoughtless and uncontrolled consumption of additives. Essential substances should be supplied to the body in sufficient, but not excessive amounts.
Vitamins. Today, there are several essential substances called vitamins. Each of the vitamins, as a rule, is a cofactor for a large number of enzymatic processes, so a deficiency of even one of them can cause a whole range of diseases.
Vitamins are divided into water soluble and fat soluble.
Vitamin C and B vitamins are water soluble. They are easily absorbed from food and do not require special carrier proteins (with the exception of vitamin B12) to be transported by the blood. An excess of these vitamins does not lead to negative consequences; it is quickly excreted by the kidneys, and vitamins B9 and B12 are excreted in the bile.
Vitamins A, D, E, and K are fat soluble. With the exception of vitamin K, they can be stored in the body's liver and adipose tissue to provide a buffer, and therefore may not be consumed as regularly as water-soluble vitamins. Fat-soluble vitamins require bile from the intestines to be absorbed into the lymphatic system.
With adequate nutrition and metabolism, the body is able to receive vitamins in the required volumes. Some vitamins can be produced in sufficient quantities by the intestinal microflora under favorable conditions for intestinal bacteria. These are mainly vitamins B1, B2, B6, B7, B9, B12, C, K, as well as nicotinic and pantothenic acids.
The problem, however, is both adequate nutrition and adequate microflora, which may necessitate temporary supplementation and/or prebiotics/probiotics. The disease state of the body, lactation, genetic abnormalities or advanced age can also cause an increased need for certain vitamins or chemical elements. In addition, a significant part of the vitamins is lost during the refining of whole foods and during the heat treatment of food.
Despite their potential benefits, vitamin supplements are generally not a good idea and should not replace healthy food as a source of essential nutrients. But just as crutches are needed for leg fractures, supplements may be needed either temporarily for nutritional vitamin or chemical deficiencies, or permanently for genetically determined deficiencies.
The norms of the physiological need for each of the vitamins for women aged 18-60 are presented in the table above. For other age groups, they may be slightly different *. The recommendations of the European Food Safety Agency * are very close to the recommendations of the US National Academy (RDA/AI) *, and the US Institute of Medicine *, which we will focus on further.
Let us note in advance that the concept of «consumption norms» for vitamins is very crafty. These rates are derived from discussion and not from any experiment or calculation *. There are no scientific methods for determining consumption rates. In fact, no one can empirically calculate how much of a particular vitamin is required to ensure all those biochemical reactions of the body in which they participate. And in those states in which the body is.
Those norms that are proposed by medical organizations are derived from consumption levels that avoid obvious vitamin deficiency and the occurrence of associated diseases, such as scurvy or beriberi in 97-98% of the population. But this does not mean at all that they will be sufficient to adequately perform some metabolic functions.
Vitamin levels in the body are usually monitored by their levels in the blood rather than by their levels inside cells, where they actually work as coenzymes for intracellular enzymes. To what extent the concentration of vitamins in the blood corresponds to their concentration inside cells remains an open question.
In addition, the recommendations of these standards do not take into account that in fact the main source of some vitamins (for example, many B vitamins) is not food, but intestinal bacteria. With a healthy colon microflora and sufficient nutrition of intestinal bacteria, supplements of such vitamins most likely will not be required. Conversely, if the microflora is unhealthy, the proposed standards may be insufficient. In other words, when determining the dosage of vitamin supplements, one must consider not only the base level in the body, but also the health of the entire body. Fortunately, the safe dose of water-soluble B vitamins is tens of times higher than the recommended levels. However, the same cannot be said about all other vitamins and vitamin-like substances.
Let's compare the recommended minimum intake of vitamins with the actual intake of our ancestors.
According to estimates, a Late Paleolithic human consumed ~ 600 mg of vitamin C daily, which is 8 times higher than the recommended amount. In comparison with modern man, he consumed more: carotene – 1.7 times; vitamin A – 2.7 times; riboflavin – 3.6 times; folate – 1.5 times; thiamine – 2.6 times; ascorbate – 8.4 times; vitamin E – 3.1 times *. While these data are not formally conclusive evidence for the need for high doses of these vitamins, they do raise questions about the soundness of the recommendations put forward by the authorities.
With regard to cancer, three periods of correction with nutritional supplements can be considered: 1) prevention; 2) time of usual treatment after diagnosis; and 3) post-treatment period.
Vitamins for prevention. Fat-soluble vitamins tend to be poorly absorbed. And water-soluble vitamins tend to be rapidly excreted from the body, which requires their constant consumption with food. Otherwise, there may first be a deficiency, and then a deficiency of many vitamins, which can increase the risk of various diseases.
• Vitamin A *. Two forms of vitamin A can be ingested through food: 1) preformed vitamin A found in animal products (eg liver, whole milk) and absorbed as retinol; and 2) provitamin A carotenoids, derived from fruits and leafy vegetables and converted to retinol after ingestion. Most supplements contain preformed vitamin A.
In older women, β-carotene is significantly inversely related to estradiol levels *. Accordingly, in premenopause, higher plasma concentrations of α- and β-carotene are associated with a lower risk of ER– breast cancer subtypes * *. In addition, γ- and δ-tocopherols are also effective natural agents for the prevention and treatment of estrogen-sensitive subtypes of breast cancer *. However, not all studies agree with this conclusion *.
Two well-known clinical studies have found a negative role for ultra-high doses of vitamin A in smokers. In the first study, supplementation of 30 mg (50'000 IU) of beta-carotene plus 7.5 mg (25'000 IU) of vitamin A (retinol palmitate) increased the risk of lung cancer by 28% in smokers, and in people exposed to asbestos and mortality by 17% compared to control *. And in a second study, 20 mg (33'333 IU) supplements of beta-carotene increased the risk of lung cancer by more than 16% in people who smoked at least 5 cigarettes a day *. Interestingly, women in the study had cancer risk, heart attack risk, and death rates twice as high as men.
The tolerable upper intake level (UL) for vitamin A is 3 mg/day, i.e. 7-10 times lower than what was applied. Thus, the negative result obtained in these studies speaks more about the dangers of an overdose of vitamin A than about the dangers of it as such. Moderate doses of retinol, unlike excessive doses of beta-carotene, do not increase cancer risk * but rather decrease it *.
Additional research has shed new light on the paradoxical effect of vitamin A. The negative effects of taking synthetic alpha-tocopherol were observed only in smokers who continued to smoke during radiation therapy. Cigarette smoking before or after radiotherapy did not affect the effects of alpha-tocopherol (400 IU/day) and beta-carotene (30 mg/day) supplementation. However, among those who smoked during radiotherapy, the risk of recurrence was 2.4 times higher, and the risk of all-cause mortality was 3.4 times higher *.
• Vitamins B2 *, B6 *, B9 * and B12 *. The results of reviews and meta-analyses show that increased intake of vitamins B2, B6 and B9 may reduce the risk of breast cancer, although in fact their effect was not significant * *. So, folic acid and vitamin B6 may reduce the risk of ER– and PR–, but not ER+ and PR+ subtypes of breast cancer. For example, an increase in folic acid intake of 100 μg/day corresponds to a 7% reduction in the risk of death from ER–/PR– breast cancer.
One reason for this remarkable association is the ability of these vitamins to keep levels of homocysteine, which is a pro-inflammatory molecule, low. The conversion of methionine obtained from protein foods into homocysteine and vice versa is carried out with the participation of vitamins B12, B9, B6. Deficiency in any of these leads to the accumulation of homocysteine, which damages cells and tissues, and an associated increase in inflammatory levels.
In a special study, the following prophylactic doses were named optimal: B2 – 3 mg/day; B6 – 2-4 mg/day; B9 – 350-550 μg/day; B12 – 8-10 μg/day. That is, twice the doses that are currently recommended *.
• Vitamin B3 (niacin, nicotinamide, niacinamide, vitamin PP) * is known as a powerful innate immune system booster to help fight antibiotic-resistant bacterial infections, including Staphylococcus aureus *. Since inflammation caused by infection is a tumor promoting factor, nicotinamide has the potential to reduce the risk of cancer. It is also suggested that niacin is an epigenetic modulator that enhances the expression of specific antimicrobial genes *.
Nicotinamide (500-1'500 mg/day) is known to prevent UV-induced skin immunity *, which reduces the risk of non-melanoma skin cancers * and increases the efficacy of topical photodynamic therapy *. Preclinical studies suggest a preventive benefit of nicotinamide also in tumors of the intestines and bladder *, pancreas *, liver *, lungs * *, as well as breast adenocarcinoma *. But clinical studies of the preventive effect of vitamin B3 in relation to breast cancer have not yet been found.
One form of vitamin B3, nicotinamide riboside (2×500 mg), has been clinically shown to improve mitochondrial respiration of cells, adding energy to them * *.
The recommended daily dose of B3 is 16 mg, which, with a healthy intestinal microflora, can be provided with adequate nutrition. As a prophylactic (in the absence of contraindications), up to 25 mg/day is allowed. However, the manifestation of the therapeutic effect mentioned above requires a much higher dose, which cannot be provided from dietary sources.
Although an overdose of vitamin B3 usually does not cause negative effects, it is not recommended to do this for a long time or as a preventive measure. The dosage of this form of vitamin, such as niacinamide, is considered toxic in excess of 3 g/day; and niacin is toxic at even lower doses, around 2 g/day.
• Vitamin C, as well as vitamin A and retinoids, significantly reduces the risk of breast cancer.
- A large-scale, multi-year study has concluded that people with higher blood levels of vitamin C are at significantly lower risk of cardiovascular disease and cancer death, and have up to a 25% lower risk of death from all causes *.
- Intake of 300 mg/day of vitamin C shows a consistent inverse relationship with breast cancer risk, especially in postmenopausal women (the relative risk for the highest and lowest quintiles is 31%) *.
- Premenopausal women with a family history of breast cancer but who consume about 200 mg/day of vitamin C in their diet have a 63% lower risk of breast cancer compared to women who consume about 70 mg/day *.
- Overweight women who consumed 110 mg/day of vitamin C had a 39% lower risk of breast cancer compared to women who consumed 30 mg/day *.
- Women with the highest intake of vitamin C prior to being diagnosed with breast cancer are 25% less likely to die from it than women with the lowest intake *.
- An estimated total intake of 380 mg/day of vitamin C can reduce the risk of breast cancer in postmenopausal women by 16% *.
The results of all these studies may indicate that modern man consumes insufficient amounts of vitamin C.
• Multivitamins. There are some indications that multivitamin supplements may reduce the risk of breast cancer in women who consume more than 10 g/day of alcohol, as well as reduce the risk of ER–/PR– tumors in all women *. However, the results of many studies on the association between preventive intake of certain vitamins and cancer risk remain either inconclusive or inconsistent *.
One of the large-scale studies (SU.VI.MAX Study) studied the effect of long-term intake of a combination of 120 mg of ascorbic acid, 30 mg of vitamin E, 100 ml of selenium, 6 mg of β-carotene and 20 mg of zinc. As a preventive measure for cancer, the supplements appeared to be more beneficial for men than for women, which was associated with men's inherently lower levels of antioxidants *. But this does not mean that all women are not deficient in antioxidants in general, and specific vitamins and/or minerals in particular.
It is also important to note that all of the studies noted above did not measure the initial level of vitamins and antioxidants in the body. It is possible that the positive effect was the result of the patients reaching the recommended level of vitamin intake, or slightly exceeding them. While a 20-30-fold excess, as was the case in both of the above studies of smokers who took vitamin A, only worsens the situation.
Indeed, a higher intake of vitamins can markedly reduce the incidence of cancer compared to a lower intake * *, but this seems to be the case in cases of their deficiency. Because vitamin supplements in dosages above the Recommended Dietary Intake (RDA) do not reduce cancer risk * * *. What's more, some studies have reported an increased risk of breast cancer in women who took multivitamins as a preventive measure *. However, the latter result requires clarification that not all vitamins are dangerous in overdose.
Based on this ambiguous theoretical basis, the American Cancer Society recommends only whole foods as a source of vitamins for cancer prevention *. Indeed, adequate nutrition, as discussed earlier †, is theoretically able to meet the physiological need for vitamins and provide the body with a variety of biologically active substances that act synergistically, which no single supplement can provide.
Unfortunately, in practice, for various reasons, even healthy food may not cover the deficiency of essential substances *, which include vitamins. Today, most people consume some micronutrients (vitamins A, B6 and C; folic acid; zinc and magnesium) from food below the recommended daily allowance (RDA).
In this case, taking vitamin supplements may be justified as a prophylactic or maintenance agent – to prevent or eliminate vitamin deficiency. This is especially important for older adults because the body's ability to both synthesize vitamin D from sunlight and absorb vitamin В12 from food declines steadily with age. Not surprisingly, about half of people over the age of 51 take supplements daily *.
Taking vitamin supplements can also be justified by the fact that not only an inadequate diet, but also an inadequate intestinal microflora, as well as many therapeutic agents, can reduce the intake of some vitamins to a level below physiological. For example, long-term use of high doses of metformin (≥ 850 mg) can reduce the absorption of vitamin B12 by up to 20% in 10-30% of patients *, which entails serious side effects, including the risk of Alzheimer's disease.
In general, as a preventive measure, supplements of one or another vitamin will certainly be useful in case of their deficiency, however, in the event of a significant overdose, they will not provide any benefit, and in some cases can be harmful. To determine the adequate dosage of any of the supplements, one should focus not so much on the recommended consumption rates, but on the laboratory analysis of their level in the body.
Despite the fact that the best vitamin complex is a plant-based food containing natural, not synthesized vitamins, taking a ready-made complex in the form of supplements allows you to control the level of substances taken. Some multivitamin complexes contain all the listed vitamins in the required amount (for example, OptiMen® for men and OptiWomen® for women), although the composition of the proposed complexes does not always correspond to the needs of a particular person.
Vitamins for healing. While multivitamin and mineral supplements may be beneficial after a breast cancer diagnosis and in breast cancer survivors * * * *, there is still no consensus among clinicians about the benefits of taking certain supplements.
It is believed that cancer cells need much more vitamins than normal cells. Some prominent cancer research organizations advise against the use of any supplement for cancer survivors * and cancer prevention *. These are, for example, the American Cancer Society, the World Cancer Research Fund and the American Institute for Cancer Research.
However, cancer is often accompanied by deficiencies in many nutrients, including vitamins. Accordingly, normal cells experience an increased need for them. It is logical to assume that an adequate supply of cells should contribute to the healing process of a chronic wound, which is a tumor. However, dosage will also be an important consideration here, because megadoses of some (but not all) vitamins can indeed not only not improve * *, but even worsen such a treatment indicator as patient survival *.
Let's also not forget about the genetically determined deficiencies of certain vitamins, which certainly requires their regular intake. In any case, vitamins in the recommended daily doses (or close to them) are not medicines, and can only be an adjuvant.
• Complexes of vitamins and minerals. A large prospective study showed that women with invasive breast cancer who took antioxidants (vitamin E, vitamin C, multivitamins) in the first 6 months after diagnosis had an 18% lower risk of overall mortality and a 22% lower risk of recurrence. This feedback was observed regardless of whether vitamins were used concomitantly with chemotherapy or not, however, it was observed only among those patients who did not undergo radiation therapy *.
In another study, patients taking high-dose supplements experienced four times longer survival than those not taking them *. Vitamin C (12 g/day), vitamin B3 (niacin, niacinamide) – 1.5-3 g/day, vitamin B6 (pyridoxine) – 250 mg/day, folic acid – 5-10 mg/day, beta-carotene – 15-30 mg/day, vitamin E – 0.5 mg/day, selenium, zinc sulfate, calcium, magnesium and other additives.
It has also been reported that the effect of multivitamin supplementation may depend on the size of the breast tumor. With a tumor size of up to 2 cm, multivitamins reduced the risk of its development, and with a size of more than 2 cm, on the contrary, they increased *.
Many vitamins work in combination, and their joint intake provides a synergistic effect. This is, for example, the combination of vitamins C and E, as well as D and K. In postmenopausal women, the combination of vitamin C (500 mg/day) with vitamin E (400 mg/day) restores antioxidant levels and reduces DNA damage in breast cancer chemotherapy *, and also protects against lipid peroxidation induced by tamoxifen treatment (at tamoxifen 10 mg twice daily) *.
The combination of vitamins C and K can increase the sensitivity of cancer cells to conventional chemotherapy * (5'000 mg C and 50 mg K3 per day *).
A combination of magnesium (100 mg), zinc (4 mg), calcium (400 mg) and vitamin D (200 IU) taken twice daily for 12 weeks by women with polycystic ovary syndrome had a strong beneficial effect on hormonal profiles, biomarkers of inflammation and oxidative stress *.
Critical vitamins for cancer. Many studies show that the tissues of cancer patients are usually depleted of certain vitamins and minerals. At a minimum, this applies to vitamins such as D and C, as well as chemical elements such as iodine, selenium and zinc. However, their deficiency is widespread among apparently healthy people, exposing them to the risk of many degenerative diseases.
In breast cancer, vitamins A, D, C, E, B2 and B9 are most often deficient.
• Vitamin A (retinol palmitate) * and its metabolites regulate the growth, apoptosis and differentiation of epithelial cells, so a deficiency of this vitamin can have tragic consequences for the development of cancer. Vitamin A deficiency can also markedly impair both innate and adaptive immunity * *, especially in relation to natural killer cell activity * *.
In addition, some studies suggest that the metabolite ATRA (all-trans retinoic acid) has antiestrogenic properties, making ER+ cells generally susceptible to retinoid treatment *.
In women with early-stage breast cancer, higher blood carotenoids are associated with a higher survival rate over the next 7 years *.
Retinoic acid is able to interfere with the DNA repair of radiation-damaged cancer cells more effectively than DNA repair of normal cells *. In patients with metastatic postmenopausal breast cancer, mega-doses of vitamin A (350'000-500'000 IU/day) taken during chemotherapy significantly increased the number of complete responses *. However, after a course of therapy, continuing to take vitamin A in such high doses is likely to only bring harm. Supplementation as low as 5'000 IU/day (1'500 μg/day) of any vitamin A other than beta-carotene may, for example, increase the risk of osteoporosis.
Vitamin A exists in five forms in the body: retinol, retinal, retinoic acid, retinyl palmitate, and beta-carotene. Each of these forms of vitamin A is important because it performs a function that other forms cannot. Retinol maintains skin health, retinal – vision, retinoic acid – skin and epithelial tissue, retinyl palmitate is needed to conserve vitamin A reserves in the liver. Beta-carotene can be broken down into any of the listed forms of vitamin A.
There are many natural sources of vitamin A. Converted vitamin A (retinyl esters) is found in some animal products, while provitamin A (carotenes) is found in dark colored vegetables and fruits, and red palm oil. The need for vitamin A with adequate nutrition is usually easily met.
The recommended daily intake of vitamin A is 1 mg, which requires at least 12 mg of beta-carotene. The Tolerable Upper Intake Level for vitamin A for adults is set at 3 mg/day *. However, studies show that beta-carotene intake below 3.4 mg/day increases the relative risk of breast cancer by at least 15% *. On the other hand, prolonged repeated overdose of vitamin A, as noted above, can also have negative consequences. In addition to the negative effects already mentioned, an overdose of vitamin A can negate the health benefits of taking vitamin D *.
Vitamin A sufficiency in the body can be reliably determined only by its reserves in the liver (up to 1'400 µg/g) using a biopsy, but such a procedure is, of course, unacceptable. Plasma retinol concentrations are tightly controlled and begin to fall only when its reserves in the liver are depleted to a level of 20 μg/g and below, which will indicate its catastrophic deficiency. Health conditions can affect plasma retinol levels, making direct monitoring of its levels problematic. For this, less accurate indirect methods are used.
• Vitamin В8 (myo-inositol, inositol hexaphosphate): up to 1-2 g/day *.
Myo-inositol exhibits a pronounced preventive anticarcinogenic effect * *, suppresses malignant transformations *, promotes the differentiation of cancer cells *, enhances the antiproliferative effect of adriamycin and tamoxifen *, radically reduces the level of C-reactive protein *, significantly reduces the metastatic ability of cancer cells * *; counteracts aggregation of red blood cells and alleviates the side effects of chemotherapy in relation to the blood count *. At the same time, its antiproliferative effect and ability to reduce the formation of colonies of cancer cells do not depend on the subtype of breast cancer *.
Patients treated with 2×3 g of a mixture of inositol and IP6 (inositol hexaphosphate) for 6 months of chemotherapy (fluoracil, epidoxyrubicin and cyclophosphamide) showed no change in the number of leukocytes and platelets during treatment *. Applying 4% inositol hexaphosphate gel to the breast also significantly reduces the side effects of chemotherapy and improves white blood cell and platelet counts *.
A complex containing boswellia (50 mg), myo-inositol (200 mg), betaine (175 mg), N-acetylcysteine and vitamins В2, В6, В9 and В12 reduces the size of fibroadenoma in premenopausal women within 6 months without showing negative side effects *. In smokers with lung dysplasia, myo-inositol (18 g/day) significantly delayed the transformation of a benign tumor into a malignant one for 6 months *. A case of 3-year remission of metastatic melanoma has also been reported after a course of treatment with IP6+inositol *.
The recommended prophylactic dose of a mixture of inositol and inositol hexaphosphate is 1-2 g/day, and the therapeutic dose for cancer is 8-12 g/day *. A daily dose of 18 g of oral myo-inositol for 3 months does not cause noticeable negative side effects *. A well-known natural source of inositol is carob bean flour (carob).
• Vitamins В9 * and В12 *. Deficiency of both the first and second are widespread among the population of «wealthy» countries. Although we usually get enough B vitamins from food, in many cases there can be a lack of B12. This can lead to a decrease in non-carcinogenic metabolites of estrogen and an increase in its carcinogenic metabolites. Deficiency of zinc, vitamins B6, B9 and B12 can lead to the same severe chromosomal damage as increased radiation *.
Higher dietary folate intake may reduce the risk of breast cancer, however the degree of risk depends on menopausal status and estrogen receptor status * *. At the same time, there are reasonable concerns about the proliferative consequences of taking vitamins B6, B9 and B12 *. For example, folate promotes DNA base methylation by affecting the expression levels of various genes (epigenetic control). This somewhat explains their complex role, which can be both beneficial and harmful. However, large-scale studies show their complex positive rather than negative role.
A direct link has been reported between low dietary folate intake and breast cancer *, however there is no consensus regarding the dosage of its supplements. The American public recommendation is 400 micrograms/day of folic acid. Some physicians even recommend increasing its intake to 2'500-5'000 μg/day * in order to reduce systemic inflammation and related diseases. However, others wisely warn against such insanely high dosages, as folate, by penetrating the blood/brain barrier, can cause convulsions at high concentrations *.
In one study, a 100 μg/day increase in folate intake was associated with a 23% reduction in the risk of dying from breast cancer *. In other studies, a total intake of 200-300 μg/day of folic acid reduces the risk of breast cancer, and 400 μg/day or more, on the contrary, increases it *. In one study, 800 mg of folic acid and 400 mg of B12 increased the risk of cancer by 20-30% *. These results, however, are disputed by a retrospective study of 23 centers in 10 countries *.
Regardless of menopausal status and hormone receptors *, 220 μg of folate daily is associated with a lower risk of breast cancer *, and more than 400 μg is associated with a higher risk *. However, these figures seem to be valid only for the countries where these studies were conducted, because the dietary intake of folate can vary significantly from region to region. However, the general rule is that both a deficiency and an excess of folate in the blood (above 15.8 ng/mL *) increases the risk of breast cancer*. High plasma folate concentrations are more dangerous for women with a BRCA1/2 gene mutation *.
All of these studies, like many others like them, looked only at the end results, without taking into account baseline levels of both vitamins. Because of this, conflicting conclusions have been drawn, resulting in no general agreement on the benefits and dosages of В9 and В12 supplements in breast cancer.
Daily intake of even 100 micrograms of methylfolate and 2 micrograms of methylcobalamin significantly reduces the risks of diseases that are associated with a defect in the MTHFR gene, observed in about half of the world's population, and reduces the level of homocysteine, one of the culprits of inflammation. Metformin *, antacids, alcohol *, high estrogen levels * somewhat deplete vitamins B12 and B6 needed for DNA methylation and estrogens, which may make it worth taking these vitamins. A strict vegan diet may be another reason to take B12 (from 5 μg/day).
A prospective study from the National Health and Nutrition Survey (NHANES) shows that adequate absorption of essential nutrients from food is more effective than absorption from supplements *. Natural food folates differ from the chemical structure of the synthetic folic acid used for food fortification, resulting in a different metabolism *. Therefore, natural folates found in food will be a better choice than folic acid.
The metabolism of folic acid to folate requires the enzyme dihydrofolate reductase. But because folic acid is a synthetic form of folate not found in natural foods, this enzyme is deficient in the human liver *. Thus, the rate of folic acid metabolism is very low, especially in people with low folic acid activity. And the benefits of its use are limited by the amount of dihydrofolate reductase that the patient's liver can provide. When using supplements, some experts recommend vitamin B12 in a methylated form (such as methyltetrahydrofolate) because a significant portion of the population has a gene defect that is associated with an enzyme that methylates B vitamins. With the methylated form, the body is guaranteed to get B12 from a supplement.
A diet high in these vegetables can eliminate the need for supplemental B vitamins. If dietary folate is deficient, supplementation is better than deficiency. Dietary sources of vitamin B12 include shellfish, meat, eggs, and dairy products. The intake of these vitamins, as well as other nutrients, can be indirectly and very approximately estimated by analyzing the composition of your diet using specialized programs such as Diet Pro *, DietMaster *, DietOrganizer *, My Healthy Diet *.
In general, folic acid 250 μg/day and methylcobalamin 5 μg/day are likely to be safe, although possibly ineffective. Again, these recommendations refer to the population of «wealthy» countries, i.e. to that group of the population where on average there is a deficiency of these vitamins. However, even within these populations there can be significant variation in the amount of supplementation required.
B vitamins are water soluble and for this reason it is very difficult to overdose on them. Therefore, you can safely take 3-5 μg of methylcobalamin daily as a supplement; the excess will simply pass into urine.
It is believed that the content of B12 in the blood should be at least 250 pg/ml *. However, just as is the case with many other substances, B12 levels in the blood do not exactly correspond to B12 levels in cells. In fact, there may be a serious underlying functional deficiency of vitamin B12, even though blood levels are normal. Direct measurement of homocysteine or methylmalonic acid levels will be a more accurate reflection of functional vitamin B12 levels.
When determining the dosage, make sure that the intake of these vitamins in the form of supplements is not excessive. As with other vitamins, you should only eliminate the lack of B9 and B12, and not exceed their physiological levels. The content of B12 in the blood must be at least 250 pg/mL *. Methylcobalamin, unlike cyanocobalamin, is the preferred form of vitamin B12. Methylcobalamin is completely ready for absorption, while cyanocobalamin requires prior metabolization with the participation of the antioxidant enzyme (glutathione). At the same time, cyanocobalamin is more stable, while methylcobalamin is much more easily destroyed, especially when exposed to light.
• Vitamin C * (L-ascorbic acid, potassium ascorbate, sodium ascorbate, calcium ascorbate): up to 100-200 mg/day.
Vitamin C is an essential nutrient and is involved in a number of critical biological processes. In particular, it takes an active part in the mitochondrial respiration of cells. The human body is unable to synthesize vitamin C and therefore needs a constant supply. The decrease in the amount of raw plant foods in the human diet over the past century has led to a significant reduction in the intake of vitamin C.
The dosage of vitamin C can have different, and even opposite, effects. So, its low concentrations in the blood act as an antioxidant, and high concentrations act as a prooxidant. Accordingly, a distinction is made between low- and high-dose vitamin C therapy.
Low dose therapy. Inflammatory and tumor processes are accompanied by the formation of large amounts of free radicals, which increases the need for healthy tissues in antioxidants such as vitamins C and E in order to reduce the oxidative load.
Any serious illness «burns» vitamin C, because it is actively spent to overcome the stressful situation that has arisen. Studies show that in cancer patients, the average level of vitamin C in plasma is lower than in healthy people * *, which manifests itself in the form of hypovitaminosis (< 23 μM) or direct deficiency (< 11 μM), and as the tumor develops, its level, as usually steadily falling *. It is significant that animals capable of synthesizing vitamin C in their liver, under conditions of tumor load, increase the level of production of their endogenous vitamin C * *, which may indicate an increased need for it in cancer.
Frequent use of vitamin C and vitamin E after breast cancer diagnosis has been associated with a reduced chance of recurrence * *. Once diagnosed, vitamin C provides a 15% reduction in the relative risk of death from breast cancer when taken as a supplement (400 mg/day), and also provides a 22% reduction when taken from dietary sources (by 100 mg/day) *. The latter is easily achieved with an adequate intake of fresh, plant-based foods, eliminating the need for vitamin C supplements.
It would be logical to assume that, due to its antioxidant activity, ascorbate may counteract the effectiveness of radiation and chemotherapy. However, meta-reviews of prospective studies do not confirm this version, and rather say the opposite *. This suggests that vitamin C may protect healthy cells from oxidative damage while not protecting cancer cells. However, there are still conflicting opinions regarding the dosage, route of administration, and appropriateness of vitamin C administration in cancer therapy.
Chemotherapy using drugs such as cisplatin *, fluorouracil *, nilotinib *, interleukin-2 (IL-2) * and several others can significantly deplete vitamin C levels in cancer patients. While plasma vitamin C concentrations usually return to baseline about one month after chemotherapy * *, they most often remain well below optimal.
In a randomized 5-month trial, supplementation of vitamin C (500 mg/day) and vitamin E (400 mg/day) restored the body's antioxidant status, which had been reduced during chemotherapy *. Other studies have reached similar conclusions after intravenous administration of vitamin C * *. Given the importance of vitamin C for cellular health, low-dose vitamin C supplementation after diagnosis seems to make sense. But still remains controversial during anticancer therapy.
In hormone therapy in postmenopausal women with breast cancer using tamoxifen, supplementation of vitamin C (500 mg/day) with vitamin E (400 mg/day) for 3 months attenuated the negative effect of tamoxifen on plasma lipid and lipoprotein levels *. Combining vitamin C with vitamin E is all the more justified because they work better together than apart; and, in addition, vitamin E is classified as a so-called mitokan, i.e. a substance that allows the rejection of non-functional mitochondria *.
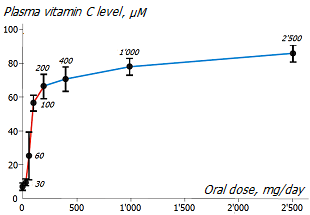
The degree of absorption of ascorbate from the intestines into the blood depends on the size of the dose taken *. Exceeding a single oral dose of 200mg of ascorbate usually does not produce a further marked increase in blood levels of vitamin C *, and makes little sense. This effect leads to the idea that this is the required level of vitamin C intake for the body.
Since vitamin C is very quickly excreted from the body (half-life ~ 1.5 hours), developed by the so-called buffered vitamin C variants. An example of a good choice is calcium ascorbate, which has neutral acidity, rapid cellular uptake, and long blood retention (~ 8 hours).
Recently, new profitable offers have appeared; for example, liposomal vitamin C, which, at the same dosage, allows you to increase its absorption by 5 times compared to pure vitamin C *.
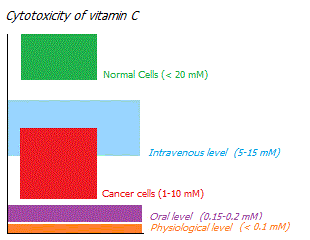
High dose therapy. Vitamin C can also be used as an independent or additional therapeutic agent, however, this will require its high concentration in the blood, which can only be achieved by intravenous administration. A high concentration of ascorbate creates large amounts of hydrogen peroxide in the intercellular space, which is detrimental to cancer cells that are deficient in antioxidant enzymes. As can be seen from the figure shown here, non-tumor cells successfully withstand a concentration of vitamin C that is toxic to cancer cells.
In addition to its pro-oxidant effect, ascorbate helps to restore aerobic respiration * by reducing the activity of the enzyme lactate dehydrogenase, which is responsible for the conversion of pyruvate to lactate *. In vitro studies have shown that ascorbate, especially in combination with selenium, promotes the return of pre-cancerous cells to normal metabolism * * *. One of the possible reasons for this was cited as cell differentiation *.
While the concentration of ascorbate in the blood can be relatively easily regulated, its entry into the cell is regulated by the cell itself. And it depends little on the extracellular concentration of ascorbate. However, high concentrations of vitamin C in the tumor microenvironment will have a detrimental effect on it. In the oxygen-rich environment of blood vessels, ascorbate is oxidized to dehydroascorbate. And dehydroascorbate exhibits oxidative toxicity under aerobic conditions. That is, in those that are observed inside the tumor, but not in normal tissue *.
Getting inside cancer cells will be less problematic for vitamin C than getting inside normal cells. Many cell types transport ascorbate in its oxidized form (dehydroascorbate) via facilitated glucose transporters *. And since cancer cells have an increased need for glucose, they increase the number of glucose carriers to meet it *.
Several clinical trials of high-dose vitamin C therapy have been conducted. In one of these, intravenous ascorbate (1.5 g/kg three times a week) was used in combination with standard chemotherapy. The study involved 14 hopeless patients, for whom oncologists predicted the possibility of a positive objective response of no more than 33%. However, in 6 patients, high-dose ascorbate therapy provided short-term or long-term stabilization of the condition with symptomatic improvement *.
Another clinical trial showed that high doses of ascorbate compared to low doses of ascorbate significantly increased the survival time of patients (246 days versus 43) *. However, other clinical trials and case-control studies under various conditions have not shown a clear benefit of ascorbate monotherapy *.
Several clinical studies show a synergistic effect of combining ascorbate with standard chemotherapeutic agents in various types of tumors*. The current recommendations for vitamin therapy include the following points: the dosage of ascorbate should be about 1 g/kg for at least 2 infusions per week and for at least 2 months, and preferably 3 months, and only after this period can its effectiveness be evaluated *.
• Vitamin D3 * (cholecalciferol): 600-2'000 IU/day (15-50 μg/day) in summer and 800-4'000 IU/day (20-100 μg/day) in winter.
One of the main uses of vitamin D is to regulate the levels of calcium, magnesium, and phosphate in the body. When active, the nuclear vitamin D receptor (VDR) affects the transcription of at least 913 genes and influences numerous biological processes *.
When it comes to breast cancer, vitamin D has a lot of potential. It regulates the expression of genes involved in the development of cancer; stimulates cell differentiation and apoptosis; inhibits proliferation, angiogenesis, invasion and inflammation; reduces the metastatic potential of the tumor; and also inhibits aromatase activity, thereby reducing the level of estrogen load * *.
Vitamin D can act in two ways: first, by penetrating into the cell nucleus, and then participating in the expression of certain genes *; and secondly, by binding to their receptors on the surface of cells, and participating in cell signaling *. It is not difficult for him to enter the cell and the cell nucleus, since in fact vitamin D is a steroid-like hormone.
In order for vitamin D to be active, cholecalciferol must first be converted in the liver to 25(OH)D, called calcidiol (25-hydroxyvitamin D). And then, mostly in the kidneys, it has to turn into 1,25-dihydroxyvitamin D, called calcitriol. At the same time, calcidiol is the form stored by the body for storage, and calcitriol is a water-soluble form actively involved in the biochemical processes of the body.
In tumor cells, vitamin D stimulates apoptosis and differentiation, and also inhibits angiogenesis and proliferation *. Vitamin D activates immune T cells and promotes their differentiation *. Vitamin D compounds also have the ability to regulate growth factor and cytokine signaling in breast cancer cells *. Vitamin D3 and its analogues inhibit the proliferative activity of IGF-1 in breast cancer cells by increasing the expression of IGF-1 binding proteins *.
In addition, vitamin D enhances intestinal absorption of phosphorus and calcium, which limits bone loss *, including that caused by tamoxifen withdrawal *. Finally, vitamin D reduces inflammation *, reduces blood vessel stiffness * to help lower blood pressure, and reduces insulin resistance *.
A distinction is made between vitamin D deficiency (< 20 ng/mL, i.e. < 50 nM/L), vitamin D inefficiency (20-30 ng/mL, i.e. < 75 nM/L), and optimal serum levels (30-80 ng/mL, i.e. 75-200 nM/L). A level above 250 nM/L is considered toxic.
Available data suggest a direct relationship between vitamin D deficiency in the body and the incidence of breast cancer. From the graph of the association of 25(OH)D concentration in the blood and the incidence of breast cancer shown here, it is obvious that the lowest incidence rate is achieved at a level of ~ 100-125 nM/L, i.e. ~ 40-50 ng/mL. In no country in the world does the average concentration of 25(OH)D in serum exceed this value. The graph data is confirmed by the results of numerous studies * * * *.
Metaanalyses show that higher versus lower levels of vitamin D correlate with a statistically significant reduction in the risk of cancer and a reduction in the risk of death in cancer patients * * *. The relative risk reduction was twice that among postmenopausal women and was independent of tumor hormone receptor status * *.
25(OH)D levels above 75 nM/L versus levels below 50 nM/L are associated with a 7-year reduction in breast cancer risk of 20% in women under 60 and 36% in women over 60 *.
Women with blood levels of 25(OH)D above 40 ng/mL, compared to women with levels below 20 ng/mL, had a 44% lower risk of breast cancer *.
And women with 25(OH)D levels above 60 ng/mL, compared to women with levels below 20 ng/mL, already had a 5-fold lower risk of breast cancer *.
In another similar study, this difference was even more pronounced – 6.8 times *. In another similar study, a plasma vitamin D concentration of > 50 nM/L, compared with a concentration of < 50 nM/L, was shown to reduce the risk of breast cancer by 3.5 times *. However, these were the most impressive figures of all such studies; other studies showed much less favorable results.
A meta-analysis shows that every 4 ng/mL increase in 25(OH)D levels linearly reduces the risk of death from breast cancer by 6%, and every 10 ng/mL increase in 25(OH)D levels decreases by 14% *. Finally, in a 4-year, double-blind clinical trial, supplementation with 27.5 μg/day (900 IU) of vitamin D and 1'500 mg/day of calcium reduced the risk of disease in postmenopausal women by 77% compared with controls *.
Vitamin D deficiency is common in women with breast cancer *, and a growing body of evidence indicates that there is a highly significant linear dose-dependent relationship between circulating 25(OH)D levels and overall survival in breast cancer patients *.
Low blood levels of vitamin D are associated not only with increased incidence of breast cancer * *, but also with more aggressive types and poorer prognosis *. In pre-existing breast cancer, 25(OH)D levels above 30 ng/mL are associated with a reduction in patient mortality by more than a third compared to levels below 20 ng/mL *.
In one study, previously treated breast cancer women with high blood levels of vitamin D had a 28% higher 8-year survival rate than women with low levels *.
In another study, women who were vitamin D deficient at the time of breast cancer diagnosis were 94% more likely to spread the tumor further, and 73% more likely to die over the next 10 years, compared to women with sufficient vitamin D levels *.
Conversely, women with high summer plasma 25(OH)D levels (above 32 ng/mL), compared to women with low levels (below 17 ng/mL), had a significantly lower risk of developing breast cancer *.
The fact that African American women have significantly higher incidence * and aggressiveness * of breast cancer than white American women may, among other things, be explained by the more UV-sensitive fair skin in white people and the corresponding higher levels of 25(OH)D in fair-skinned American women.
Meta-analysis data show that at 30 ng/mL, the 9-year post-diagnosis mortality rate is about half that of 17 ng/mL, the median level seen in US breast cancer patients at diagnosis *.
In women with invasive breast cancer aged 50-80 years, continuous supplementation of more than 400 IU/day of vitamin D after diagnosis as early as one year was associated with a marked reduction in mortality *.
Breast cancer is one of the few cancers diagnosed seasonally, with the highest rates of diagnosis in the spring and fall. The production of vitamin D from solar ultraviolet radiation is thought to reduce the risk of breast cancer in summer, and higher concentrations of melatonin reduce the risk in winter *.
25(OH)D deficiency is associated with more than just breast cancer risk. A meta-analysis of observational studies reported an inverse correlation between serum 25(OH)D levels and incidence of 12 types of cancer. An analysis of 25(OH)D cancer rates suggests that reaching 80 ng/mL (200 nM/L) versus 10 ng/mL (25 nM/L) will reduce cancer rates by ~70% *.
A large 10-year cohort study showed that individuals with vitamin D deficiency (30-50 nM/L) or profound vitamin D deficiency (< 30 nM/L) had a 1.17-fold and 1.71-fold higher overall mortality, respectively, compared with with persons who do not have its deficiency (> 50 nM/L). Vitamin D deficiency was associated with higher cardiovascular mortality (1.39 times), as well as mortality from cancer (1.42 times) and from respiratory diseases (2.5 times) *. An association between serum vitamin D concentration and cancer mortality has been found even in those meta-analyses that did not support an association between vitamin D and cancer incidence * *.
The negative relationship between serum 25(OH)D concentration and all-cause mortality was found to be non-linear, increasing sharply at 25(OH)D concentrations < 75 nM/L *, and coinciding with the threshold for increasing breast cancer incidence. Vitamin D deficiency is associated not only with cancer, but also with type II diabetes *, Alzheimer's disease *, dementia * and multiple sclerosis * *. The risk of hypertension also increases with a decrease in serum 25(OH)D levels. Individuals with vitamin D deficiency (< 75 nM/L) are twice as likely to be at safe levels (> 100 nM/L) *.
Vitamin D inefficiency is a serious global problem * *. Vitamin D inefficiency occurs in 70% of the European population * *. In France, for example, about 80% of the population is deficient in the vitamin, and 35% is deficient; and by the end of the winter period, these figures become even more dramatic. Only about 20% of French adults have a serum 25(OH)D level above 30 ng/mL *, and things are even worse in Belgium, Germany and Switzerland. A study of nearly 56'000 people in Europe found that 40.4% of them were deficient in 25(OH)D, especially people with darker skin *. Worldwide, 37.3% are deficient in 25(OH)D, and its status has not been shown to be dependent on geographic latitude *. Residents of many African countries have the same deficit as residents of European countries, if not even more *.
Adequate levels of vitamin D are especially important during puberty, when breasts are developing, and from menarche to first pregnancy, when breast cells are not fully mature. Teenage girls with the highest blood levels of vitamin D have a 50% lower risk of breast cancer in adulthood than girls with the lowest levels *. Here, adequate intake of vitamin D was estimated at 25 μg/day, i.e. one and a half times higher than the recommended norm.
The ability of the skin to synthesize vitamin D has been steadily declining year by year. At the age of 60, its production drops by 4 times compared to the age of 20, so as the body ages, the need for vitamin D supplements increases. The need for vitamin D intake also increases with inadequate absorption, dark skin color, insufficient solar exposure, as well as with an excess of fat reserves in the body. Many infectious diseases can suppress vitamin D receptors, blocking its absorption. Insulin resistance, overweight, lack of bile acids also impair the absorption of vitamin D.
In addition, chemotherapy reduces the level of vitamin D in the body *, forcing us to compensate for its deficiency through supplements *. Finally, some genetic polymorphisms can impair the body's ability to produce vitamin D. For people with these genetic characteristics, taking vitamin D is a matter of life.
Vitamin D can be replenished endogenously – by producing it in the skin under the influence of ultraviolet radiation (~ 295-315 nm), or exogenously – by eating foods high in vitamin D, or by taking specialized supplements. The first dietary intake of vitamin D is preferable *, however, dietary sources may not be sufficient to replenish the body's stores of vitamin D, because it will have to be consumed in excessive amounts. For example, to ensure adequate levels of vitamin D, you will need to eat about 400 g of fatty herring daily, and this can lead to other imbalances in the body. Sunlight is the natural and best way to increase vitamin D levels in the body.
Sunlight also allows for the natural control of other hormones such as melatonin, serotonin, calcitonin, and melanocyte-stimulating hormone; regulate circadian rhythms, reduce overall inflammatory levels and favorably affect the immune system. In the summertime, 20 to 30 minutes of daily midday sun exposure to the whole body can result in white people excreting approximately 10'000 IU of vitamin D *, which can be stored in adipose tissue for the winter. However, if only the face and hands are exposed to the sun, the production of vitamin D will be 20 or more times lower, which will obviously not be enough for its adequate synthesis.
It is widely believed that direct sunlight should be avoided in order not to provoke the appearance of melanoma or skin aging. Of course, radiation burns are carcinogenic to the skin and should be avoided. However, sunscreen is only needed as a temporary protection for untanned skin at the beginning of the summer season. Their constant use reduces the production of vitamin D, which is a carcinogenic factor for all other organs.
In fact, the fear of solar radiation has no rational basis. If solar radiation does not cause burns or skin irritation, it is unlikely to be harmful. In addition, there is no need to stay in direct sunlight; long enough subdued or diffused light. After all, statistics show that the risk of dying from skin cancer due to excessive sun exposure is several times lower than the risk of dying from breast cancer caused by vitamin D deficiency due to insufficient sun exposure.
In winter, an alternative to the natural exposure to ultraviolet radiation can be a visit to the solarium. However, it is worth noting that exposure of the body to bright light (natural or artificial) should occur in the morning in order to follow the natural circadian day:night cycle.
In cases where ultraviolet irradiation under the sun or in a solarium is either insufficient or impossible, and dietary sources of vitamin D are not enough, then supplements have to be used to eliminate its deficiency. However, exogenous sources of vitamin D do not solve the problem of sunlight deficiency, which has a wider physiological effect than just the synthesis of vitamin D *.
Vitamin D is best taken at breakfast, ie. during a meal in which the highest amount of fat is recommended. This can greatly improve its absorption. In its fat-soluble form, vitamin D can be taken once a week (a single weekly dose).
There are two main forms of vitamin D available: D2 (ergocalciferol) and D3 (cholecalciferol). Vitamin D2 is synthesized on an industrial scale by light irradiation of yeast; and it must be converted by the body to D3, which is not always the case. And vitamin D3 under the action of irradiation is synthesized by the skin of animals, and is also found in animal food, and is biologically identical to human.
Vitamin D2 has little effect * * and high doses can cause adverse side effects such as hypercalcemia, while D3 does not seem to cause side effects even at higher doses. When various forms of vitamin D were evaluated separately, only vitamin D3 was statistically significant in reducing all-cause mortality * *.
Naturally, the question arises about the dosage of vitamin D supplements. The recommendations of various experts differ several times. Be that as it may, the main indicator that determines the dosage of vitamin D should be its concentration in the blood. The Endocrine Society considers the optimal concentration of 25(OH)D in adults to be at least 75 nM/L (30 ng/mL) and recommend vitamin D supplementation at a dosage of 1'500-2'000 IU/day, but no more than 10'000 IU/day; although for obese people the rate should be increased *.
At the same time, the possibility of accumulation of vitamin D in the body appears when the value of 25(OH)D in the blood is not lower than 40 ng/mL *. In addition, a value of 50-55 ng/mL (125-140 nM/L) seems to provide the best immune resistance against viruses; in particular against SARS-CoV-19 *. However, it apparently should not significantly exceed 100 ng/mL *. The 2020 Special International Consensus Conference considered 125 nM/L to be the upper acceptable level *.
It is interesting to note that primates (baboons, gorillas, chimpanzees) kept in European zoos also have insufficient levels of vitamin D (30-40 nM/L). Moreover, this deficiency cannot be compensated for by additives. After these chimpanzees were moved into the sun in Hawaii, their average 25(OH)D levels rose to 97 nM/L *. Average 25(OH)D levels in primates in the wild vary from species to species. For example, in baboons it is 150-315 nM/L. However, this does not mean that such a value should be the target for a person.
So the 25(OH)D level to aim for is 40-60 ng/mL (100-150 nM/L) *. It is this average level that the indigenous inhabitants of the equatorial zone of Africa * * achieve, while the inhabitants of Ukraine have an average of about 12 ng/mL *, i.e. actually experiencing a serious shortage. In conditions where natural solar radiation is the main source of vitamin D, reaching a level of 50 nM/L in winter requires a level of 100 nM/L in summer * *.
How can one determine the dosage of supplements to eliminate this deficiency?
Before discussing dosages, let's note that vitamin D produced under the influence of ultraviolet light and vitamin D obtained in the form of orally supplements have different biological values. This fact may be the reason for the discrepancies in the conclusions of studies that did not distinguish between them. The transport and processing mechanisms from these two sources are quite different. Excessive intake of vitamin D supplements (> 10'000 IU/day) can cause hypercalcemic toxicity, while endogenous production under ultraviolet radiation does not pose any negative consequences. Animal experiments have shown that long-term oral intake of vitamin D in apparently non-toxic amounts causes atherosclerosis in large arteries *.
This, however, does not speak not about the dangers of vitamin D itself, but about the need to get it naturally – from sunlight. And only when the achievement of the required concentration of 25(OH)D in the blood cannot be obtained in this way, one has to agree with its intake in the form of supplements. Because its deficiency can have more detrimental consequences. At the same time, the best route of administration of vitamin D supplements is transdermal, that is, the closest to the natural route of its intake. The pharmaceutical industry has already launched the production of creams with cholecalciferol.
A more precise determination of the need to take supplements and their dosage for each specific case can only be done after analyzing the level of 25(OH)D in the blood serum. As a rule of thumb, 1'000 IU/day of vitamin D results in an increase in 25(OH)D concentration of about 10 ng/mL, although this relationship is not linear. Thus, to reach the minimum level (30 ng/mL), the average person would need to take an additional daily intake of approximately 1'000 IU of vitamin D, i.e. 2.5 times higher than the officially recommended daily dose. Supplementation with 2'000 IU of vitamin D is required to achieve optimal levels (40 ng/mL), and at least 4'000 IU to achieve safe levels (60 ng/mL), which is expected to halve the incidence of breast cancer *. For understanding, 100 μg (4'000 IU) of vitamin D can be obtained by exposing the whole body to the bright summer sun for at least 10 minutes a day, or by eating 100 g of cod liver in winter.
But these calculations referred to the average representative of the population. A more accurate determination of the need for supplements and their dosage for each specific case is possible only after analyzing the level of 25(OH)D in the blood serum. To calculate the required dose of vitamin D, you can use the following methodology: from the target level (60 ng/mL), you need to subtract the current level and multiply the resulting value by 100. For example, if the current level is 20 ng/mL, then 60-20=40, 40×100=4'000; that is, the required dosage of vitamin D will be 4'000 IU.
This calculation is applicable to most healthy people. Older people, overweight people, and rarely exposed to the sun, will probably need to increase the dose. The VITAL study found that normal-weight people who received 2'000 IU/day of vitamin D ultimately had a 24% lower incidence of cancer over five years *. However, people who were overweight or obese did not have any benefit from taking vitamin D due to their higher vitamin D requirements. Thus, overweight people may require a dose 2-3 times higher (3'000-6'000 IU/day) to achieve the same concentration in the blood. In addition, there are genetic and acquired diseases associated with dysregulation of vitamin D metabolism that can alter vitamin D requirements *.
Serum 25(OH)D levels are monitored every 3 months from the start of calciferol intake to avoid both deficiency and excess of vitamin D; and adjust the dose as soon as the optimal level is reached. Unfortunately, the problem with testing blood levels of vitamin D is that the same sample processed in different laboratories can give a wide range of results * *. This circumstance, of course, greatly reduces the information content of the analysis.
Although vitamin D is less toxic than other fat-soluble vitamins, its excess can be just as dangerous as its deficiency. It has previously been reported that serum 25(OH)D levels greater than 60 ng/mL (150 nM/L) are beginning to be associated with increased all-cause mortality in hospitalized patients *. An updated meta-analysis shows that the best all-cause mortality rates are achieved at serum 25(OH)D concentrations of 90-150 nM/L *. Such concentrations are not considered toxic *. However, problems will inevitably arise at concentrations > 250 nM/L, which can be achieved with constant daily doses in excess of 10'000-20'000 IU (depending on baseline).
Fortunately, it is extremely difficult to get an excess of vitamin D from ordinary foods or from the sun. Therefore, this caution applies rather to the supplementation of forms of vitamin D such as ergocalciferol (D2), calcidiol, or calcitriol. Their assimilation grows linearly with their consumption; whereas with an increase in consumption of the commonly prescribed cholecalciferol, its absorption deteriorates, naturally limiting the intake of vitamin D in the body.
High-dose vitamin D toxicity can be caused by vitamin A *, K *, E deficiencies, as well as calcium, magnesium, and selenium deficiencies *. Magnesium, for example, activates vitamin D obtained from food/supplements or produced by exposure to sunlight, and transports it throughout the body * *. Without enough magnesium (which is very common), acquired vitamin D can be «dead».
The bioactive form of vitamin D activates the GLA * matrix protein, which prevents vascular calcification. However, activated GLA requires vitamin K to provide this effect. A deficiency in either vitamin D or vitamin K prevents this protein from working properly. Thus, a high concentration of vitamin D with a lack of vitamin K creates a risk of calcification of soft tissues and blood vessels.
The latter fact makes us pay attention to the fact that the study of the effect of any vitamin by itself, without taking into account its joint action with other vitamins and minerals, may turn out to be incorrect *. Supplementation of any of these without the addition of its metabolic assistants may not produce the effects that were expected.
• Vitamin K *: up to 100 μg/day. There are two forms of vitamin K. Vitamin K1 (phylloquinone) is found in greens and green vegetables (parsley, brauncol, spinach, broccoli) and vegetable oils (olive). Vitamin K2 (menaquinone) is produced by gut bacteria from K1 during the fermentation process and is also found in animal products (egg yolks, liver, brain, milk fat) and fermented foods (natto, sauerkraut, full fat cheese and yogurt) *. Physiologically, both play different roles in the body, but only K2 seems to have the therapeutic efficacy of interest * *.
Vitamin K2 induces differentiation and apoptosis in a wide range of human cancer cell lines. It induces apoptosis by increasing oxidative damage to mitochondria caused by an increase in the intracellular concentration of ROS. These are mainly those ROS that are involved in apoptosis – superoxide and hydrogen peroxide. Because cancer cells are deficient in antioxidant enzymes such as catalase *, superoxide dismutase * and glutathione peroxidase *, vitamin K selectively kills cancer cells, but not normal cells * *. For the same reason, the proapoptotic action of vitamin K can be reversed by antioxidants such as catalase * or α-tocopherol *, thus preserving the viability of cancer cells.
Man has evolved under conditions of a sufficiently large intake of vitamin K from food and, perhaps, therefore, it is poorly absorbed and accumulated. Rich vegetable sources of vitamin K are kale, turnips, spinach, kale, broccoli *. The diet of a modern person in developed countries is poor in greens, which contributes to insufficient intake of vitamin K in the body, in many cases making it justified to take its supplements.
In the body, vitamin K acts synergistically with vitamin D. Without adequate amounts of vitamin K, vitamin D at recommended doses may not reach its full potential. Vitamin D supports mineralization and healthy bone structure, and vitamin K is a key factor in the metabolism of bone proteins * * *. While vitamin D provides sufficient calcium in the blood, vitamin K ensures calcium is stored in bones and teeth, but not in soft tissues such as blood vessels. The recommended weight ratio of ingested vitamin D to vitamin K2 is approximately 2.5:1, i.e. 100 micrograms of vitamin K2 per 10'000 IU of vitamin D.
A number of in vitro studies have reported a strong anti-cancer effect of the combination of vitamin C and provitamin K3 (menadione) at a molar ratio of 100:1, i.e. at their weight ratio of 40:1. Together they form a strong redox couple capable of preventing mitochondrial dysfunction, restoring oxidative phosphorylation, modulating redox homeostasis, eliminating hypoxia, and inducing apoptosis and cancer cell death *. Significantly, this combination was able to prevent the re-growth of glioma cells after the cessation of the action of the main therapeutic agent *.
A characteristic feature of apoptosis induced by the combination of vitamins C and K is its slow nature; It takes at least 3 days to see results. Perhaps this is due to the fact that it takes time for the concentration of vitamin C to reach a critical level in tumors that are prone to its accumulation * * *.
In mice, administration of a combination of vitamins C and K clearly enhanced the effect of all 6 chemotherapy drugs studied in liver tumors *, leukemia, and carcinoma *. In addition, pretreatment of mice with a combination of vitamins C and K caused a statistically significant increase in the efficacy of radiotherapy *. General and local toxicity accompanying cancer chemotherapy did not increase *.
Although proposals to use such therapy in addition to radiation or chemotherapy were first made about 20 years ago, publications on clinical studies on this topic have not yet been found. In preclinical studies, the synergy of both vitamins made it possible to reduce their concentration by 2.5-5 times to achieve the same antiproliferative effect as when using each of them separately *. Here, the concentrations of vitamins in the solution approximately corresponded to those that can be achieved by ingestion of 1 g of vitamin C and 40 mg of vitamin K3; however, since both are rapidly metabolized, this will require frequent in vivo administration.
Despite the fact that this dose of vitamin K is hundreds of times higher than the recommended one, it is not toxic even in such quantities. Dosages of 45 mg/day have been used in a number of successful clinical studies evaluating the effect of vitamin K on bone strength *. The only caveat is that synthetic pro-vitamin K3 can cause damage to liver cells * and should probably be replaced with vitamin K2. In a pilot project, a vitamin K2 analogue (45 mg/day) markedly reduced disease recurrence * and improved survival in patients with hepatocellular carcinoma *.
Based on this, we can conclude that if dietary intake of vitamin K can be sufficient for the prevention of the disease, then ready-made K2 supplements will have to be used for therapy. Since vitamin K is involved in the blood clotting process, supplementation by patients using anticoagulants needs the approval of the attending physician.
The effectiveness of vitamin supplements depends on many factors.
Deficiency of one or another vitamin can be caused by many other reasons, except for its insufficient intake from the outside. For example, a lack of vitamin B3 can be caused by a lack of tryptophan, iron, vitamins B2 and B6. Decreased levels of vitamin (actually, a hormone) D can occur due to insufficient solar exposure of the body; due to taking cholesterol-lowering drugs (statins); as well as chronically high insulin levels and inadequate hydroxylation of dietary vitamin D.
Finally, the deficiency of enzymes that provide internal synthesis of vitamins may be the result of individual genetic characteristics. However, the latter cause of vitamin deficiency is statistically much less likely than the cause of their lack in food.
In addition, the peculiarities of assimilation of the supplements taken play an important role. Consider vitamin C as an example. Its concentration in human plasma and tissues is strictly controlled, including through the activity of assimilation. At an oral dosage of 100 mg/day, absorption and satiety of vitamin C are high, and when the dosage is increased above 200 mg/day, they increase very little * *. So if the initial concentration of vitamin C is close to saturation, then oral supplementation is unlikely to produce a noticeable change in health indicators *. The body simply refuses to accept them by absorption through the intestinal wall. However, intravenous administration of vitamin C makes it possible to achieve many times greater blood saturation with it than oral administration.
Free radicals, which vitamin C works against, play a dual role, which depends on their concentration in body tissues. At low levels, they don't seem to have any noticeable effect; at moderate levels, they can contribute to the initiation and development of the tumor process; and at very high levels they become selectively cytotoxic to tumor cells *. Accordingly, under each of these conditions, the same antioxidant supplements, including vitamin C, even at the same dosage, will show completely different effects, and even more so at different dosages.
From this perspective, all research attempts to determine the optimal dosage of antioxidant, vitamin or mineral supplementation without taking into account individual characteristics of an individual sin with a serious methodological error – measuring their consumption instead of measuring their concentration in tissue. Therefore, the fundamental conclusions of these studies may be contradictory and incorrect, and the recommendations arising from them on the use and dosage of antioxidants are very approximate.
The above recommendations for vitamin D, vitamin B9, iodine, and selenium supplements are no exception. They are based solely on the widespread deficiency of these substances, and their recommended dosage is only of a general and indicative nature. The basis for taking supplements may be a deficiency in the body of one or another vitamin, determined by laboratory analysis.
The costs associated with taking vitamins can be significantly reduced if you buy certified raw materials by weight, and pack them yourself using a jewelry scale, a manual encapsulator and gelatin capsules. This solution at the same time allows you to make a recipe for a complex of vitamins and other supplements individually for each user.
Chemical elements. An abnormality of the main elements such as O, C, H, N, Ca, P causes electrolyte abnormalities, and an abnormality of 10 trace elements such as Fe, F, Si, Zn, Sr, Rb, Br, Pb, Mn, Cu (of the order of one millionth) and 14 ultra-microelements such as Al, Cd, Sn, Ba, Hg, Se, I, Mo, Ni, B, Cr, As, Co, V (of the order of one billionth), leads to functional disorders of enzymes and physiologically active substances in the body * *.
Minerals make up only 4% of the total body composition, of which 2% is calcium, about 1% is phosphorus, and the remaining 1% is all other minerals combined. The physiological requirement of each of the minerals for women aged 18-60 * are given in the table below. However, they seem to reflect the levels required for the absence of overt symptoms of disease rather than the levels required for complete health.
It seems unlikely that humans differ by an order of magnitude from other higher primates in micronutrient requirements. The roughest estimate, in terms of human scale, shows that primates in the wild consume with food: 20 times more calcium, 5 times more phosphorus, 10 times more potassium, 2 times less sodium, chlorine – 4 times more, magnesium – 15 times more, non-heme iron – 10 times more, and manganese – 40 times more than the recommended norms for humans * *.
In comparison with modern man, Late Paleolithic man consumed with food: calcium – 1.7 times more; zinc – 2.7 times more; potassium – 3 times more; sodium – 7 times less *. Even if we assume a significant excess of mineral intake, and their poor digestibility due to the large amount of fiber, such figures lead to the conclusion that the norms recommended by the healthcare system may be underestimated from the real need of the human body. And that they can eliminate the risk of diseases caused by a clear deficiency of essential substances for the majority of the population, but not provide those values at which the body feels comfortable.
Despite this, it is assumed that with an adequate diet, we should receive a sufficient amount of chemical elements from food. At the same time, there are regional features associated with a deficiency or excess of certain elements in the soil, which is reflected in their content in plant foods. And consequently, on their entry into the body.
A large number of critical elements are found in vegetables and cereal shells (germ and bran). However, the refining of cereals during their processing significantly reduces their micronutrient content. After peeling, the grains lose their germs and shell, therefore they contain on average 78% less fiber, 74% less E and B vitamins, and 69% less important elements such as zinc, selenium, calcium and potassium.
In addition, over the past half century, the intensification of agriculture has led to the mineral depletion of soils. And as a result – to the depletion of the crop with nutrients. For this reason, even a completely plant-based diet leaves the danger of insufficient micronutrient supply *. This problem is especially true for the elderly. Insufficient plant foods in the diet or restrictive diets can aggravate the situation to the point that it requires supplementation.
The greatest risk of being deficient in elements such as iodine, zinc, magnesium, potassium and selenium. Manganese, copper and zinc are part of a group of antioxidant enzymes called superoxide dismutase. Chromium, cobalt and copper, along with iodine, selenium, iron and zinc, are involved in the healthy functioning of the thyroid gland, which plays a leading role in the hormonal balance of the body. But speaking of the danger of a lack of these elements, let's not forget about the danger of their excessive excess.
• Potassium (K): up to 1-2 g/day *, and sodium (Na) are the two most abundant alkaline elements in the body.
The potassium ion is the main intracellular cation of the tissues of various organs, and is involved in the most important functions of the cell. And the sodium ion is the main extracellular cation. Thus, both of these elements are a kind of biochemical antagonists.
The recommended daily intake for potassium is ~ 5'000 mg and for sodium ~ 1'500 mg. However, the optimal dietary intake of potassium may actually be much higher than the current recommended intake. In 159 retrospective studies of the diet of Stone Age humans, potassium intake averaged 400 mEq/day, more than 4 times the current and recommended intakes * *.
According to modern concepts, the optimal ratio of sodium to potassium from food and water should be at least 1:3.5. However, under many conditions (for example, excessive sweating, diuresis, lack of vegetables in the diet, excess dietary salt, taking certain medications), this balance can be easily upset in favor of sodium, because the body partes with potassium much more easily than with sodium.
Paleolithic hunter-gatherers consumed approximately 11 grams of potassium each day from fruits, vegetables, leaves, flowers, roots, and other plant sources, and approximately 700 mg of sodium. Thus, the ratio of sodium:potassium in their diet was 1:16. The modern human diet contains more sodium (3'400 mg/day) than potassium (2'500 mg/day), and their ratio is approximately 1.36:1*. Thus, compared to the Paleolithic period, it is inverted and changed 20 times!
Assessing the sufficiency of potassium in the body is as difficult as magnesium, because they are mainly found inside the cells. And since the level of potassium storage in tissues weakly correlates with its saturation in the blood, a blood test is not able to objectively assess its reserves. If the concentration of potassium begins to decrease already in the blood, then this most likely means that it is even more lacking in the tissues, and it begins to be excreted from the blood into the tissues. Therefore, it would be better to preliminarily assess the saturation of cells with potassium by the acidity of saliva. However, more sophisticated lab tests will be needed to get an accurate picture.
Unfortunately, the modern diet is very deficient in vegetables, which could provide adequate levels of potassium. At the same time, data from experimental and observational studies show numerous positive effects of potassium supplementation. In particular, potassium reduces the risk of stroke * *, normalizes blood pressure * * and heart rate *.
With a sufficient level of consumption of vegetables, potassium deficiency is not observed. At the same time, there is usually some excess of sodium supplied with table salt, which creates an imbalance between potassium and sodium, and gives grounds for the recommendation to limit salt intake to 3-4 grams per day (2 pinches) or even less. The main food source of potassium is vegetables (potatoes, legumes, bananas, tomatoes *), and the main food source of sodium is table salt.
Thus, sodium supplements are usually not needed, unlike potassium supplements. An adequate supply of potassium is easily ensured by potassium-rich vegetables. If you need to take supplements, then when choosing between different sources of potassium, it is better to choose those that provide a slow release of it. The elimination of potassium deficiency will be more effective with the simultaneous addition of magnesium *, as well as aspartic acid, which has a pronounced ability to increase the permeability of cell membranes for magnesium and potassium ions.
For eating, you can use potassium carbonate; other acceptable sources of potassium are potassium citrate, gluconate, carbonate and phosphate. However, vegetables remain the best source of potassium.
• Calcium (Ca): up to 600 mg/day *.
Calcium is an alkaline earth element. The calcium ion Ca2+ is the second most abundant extracellular cation.
Calcium is the most abundant mineral present in the human body, indicating its many-sided, vital role. Almost all of the body's calcium is concentrated in bone tissue, but other tissues also require it. Calcium takes part in the hormonal, enzymatic, nervous, muscular and digestive systems. In addition, the circulatory and immune systems also require sufficient calcium for their successful functioning.
When the level of calcium in the body is satisfactory, calcium from food is deposited with the help of calcitonin for long-term storage in bone tissue. However, when blood calcium levels drop, such as due to chronic acid stress, calcium is drawn from the bones into the bloodstream to replace the lack of calcium. If at the same time there are problematic (for example, inflamed) places, they capture and retain calcium, which leads to local calcification. The result can be arthritis, atherosclerosis, calcifications (including in the mammary gland), hypertension, kidney stones and many other diseases.
Insufficient calcium intake is observed in some countries, especially in Southeast Asia (Thailand, China, Indonesia, Vietnam, Malaysia, Philippines) *. Residents of these regions may need calcium supplements. Many studies show some association of calcium with breast cancer risk *. Thus, a dietary intake of 1'250 mg/day of calcium slightly reduces the overall risk of cancer * and, in particular, the risk of breast cancer * in women. A daily total intake of 600 mg calcium with 400 IU vitamin D (providing 30-50 ng/mL serum vitamin D) can significantly reduce the risk of breast cancer in premenopausal * women, but not postmenopausal women * *. However, many other studies do not find benefit from calcium supplements * * *. Unfortunately, the studies looked at serum calcium levels without taking into account magnesium.
In most European countries, dietary intake of calcium satisfies the norm *, which makes calcium supplementation questionable for the vast majority of the population, no matter how well-intentioned. There remains, however, the problem of ensuring a balance between calcium and magnesium, since calcium is in some way a biochemical antagonist of magnesium.
It is problematic to achieve the required Ca:Mg ratio only by varying the modern diet. Among plant sources, few can provide an adequate balance of Ca:Mg. Thus, small calcium supplements may be justified if the body really needs calcium, or to ensure a Ca:Mg balance. The need for intake and dosage can be roughly estimated using appropriate programs such as «My Healthy Diet» *.
However, even adequate dietary intake of calcium may not be enough if there is a lack of vitamin D in the body, which enhances calcium absorption in the intestines; as well as with a lack of protein, magnesium, testosterone, vitamin E; or with an excess of phosphorus and other acidic substances *. In the same way, sufficient supply of zinc, selenium, sulfur, fluorine, vitamins A, C, E, F and group B is necessary. This once again indicates the importance of an integrated approach to eliminating mineral and vitamin deficiency in the human body.
Maximum absorption of calcium supplements is achieved at a single dose of 500 mg *. Do not forget about the sufficiency of magnesium, because without it, calcium is poorly absorbed by the body. When assessing daily calcium intake, one should take into account its intake from food. The richest dietary sources of calcium are poppy, sesame, chia, seaweed, fish with bones, dark green leafy vegetables, fermented milk products *.
Calcium carbonate has the highest concentration of calcium of any calcium salt, but for optimal absorption, it is recommended to take it with meals *. Calcium absorption improves when taken together with vitamin D, magnesium, silicon, and vitamin K2. A high intake of plant foods containing fiber, phytic and oxalic acids can significantly impair the bioavailability of calcium supplements. In addition, absorption of calcium from the intestines is usually impaired in older people. Any of these reasons may necessitate an increase in the dose taken.
Women need more calcium than men, but even if calcium is deficient, there is little point in increasing calcium supplementation above 500 mg/day and total calcium intake above 1'000 mg/day *. Excess calcium reduces the level of magnesium and active vitamin D in the blood * *. Excess calcium can easily occur even with moderate consumption of dairy products.
In addition, unbalanced calcium overload negatively affects mitochondrial function. Excessive intake of calcium leads to the fact that it begins to be deposited in unwanted places. A systematic excess of calcium or a violation of its metabolism can cause kidney stones, calcification of many organs and the vascular system, leading to visual impairment and an increased risk of stroke and heart attack.
• Magnesium (Mg): 300-500 mg/day magnesium taurate, magnesium glycinate, magnesium bicarbonate.
Magnesium is another alkaline earth element, a natural physiological calcium antagonist. The magnesium ion is the fourth most common cation in the body and the second most common intracellular cation *.
The role of magnesium is extremely important. Almost all biochemical reactions in cells require energy expenditure, and the magnesium ion Mg2+ is involved in the production of cellular energy and in the transfer of energy to reacting molecules – enzymes and coenzymes.This energy is generated by the removal of the outermost phosphate group from the ATP molecule. Being in complexes with ATP, the magnesium ion ensures the release of energy through the activity of Mg2+-dependent ATPases, which cleave the phosphate group from ATP (release energy), and also create unique conditions for the chemical reaction of its reattachment (charge the molecules with energy) *.
Magnesium ensures the control of both of these processes. It additionally binds the last two phosphate groups and ensures that the last phosphate group of ATP is split off exclusively by the enzyme, and not randomly. Thus, a magnesium deficiency can disrupt the normal course of production and expenditure of cellular energy. ATP that is randomly broken down can activate uncontrolled biochemical reactions, and on the other hand, a deficit of energy for vital biochemical reactions can occur.
Magnesium helps to keep the level of active vitamin D in the optimal range. With a lack of magnesium, any attempt to replenish the loss of potassium will be ineffective *. In general, magnesium is involved in more than three hundred vital enzymatic processes associated with energy metabolism, protein and nucleic acid synthesis *. Serum magnesium concentrations are linearly and inversely associated with the risk of total CVD events *. In addition, adequate magnesium levels counteract premature brain aging. Without magnesium, life would be impossible.
However, magnesium deficiency is widespread. For example, in Belgium, the average intake of magnesium is 270 mg per day *, which is half the recommended amount. In the US, ~ 64% of men and ~ 67% of women are deficient in magnesium. And at the age of over 71, these values reach, respectively, ~ 81% and 82% *. Insufficient consumption of vegetables and whole grains, softening and filtering drinking water, intense loss of body water, stress and alcohol consumption seriously reduce magnesium stores in the human body. Magnesium deficiency is aggravated by high calcium intake, mainly from dairy products.
Higher dietary magnesium intake in women with breast cancer was associated with a lower risk of all-cause mortality, especially in postmenopausal women. Patients with the highest magnesium intake had half the mortality rate compared to those with the lowest magnesium intake *. This effect was most pronounced in postmenopausal women and in women with a high Ca:Mg ratio.
In Egyptian women with breast cancer, magnesium deficiency was observed in 49% of cases versus 4% in the control healthy group *. The degree of decrease in serum magnesium positively correlated with the progression of the stage of malignancy *. In addition, high serum magnesium levels (1.78 mM) versus low levels (0.24 mM) are associated with a 40% reduction in the relative risk of all-cause and cardiovascular deaths, as well as a 50% reduction in cancer deaths *.
The reference values of the magnesium level in the blood serum are at the level of 0.7-1.0 mM, but, apparently, one should be guided by an indicator not lower than 1.1 mM, i.e. 2.7 mg/dL. The fact is that the analysis of the level of magnesium in the blood is not informative enough. In the blood, a satisfactory level of magnesium can be observed when it is depleted in the main places of accumulation – mitochondria, muscles and bones. Thus, the lower threshold of the «normal» level of magnesium in the blood serum may indicate not its deficiency, but its extreme deficiency in tissues and mitochondria, where it is involved in the process of cellular energy production *. To reliably determine the sufficiency of magnesium in the body, more complex studies will have to be carried out (for example, magnesium in saliva, in daily urine, in hair, as well as in whole blood, plasma and blood serum, and separately in red blood cells). Als we de toereikendheid van de magnesiuminname willen bepalen, kunnen we het beste de mate van uitscheiding in de urine beoordelen (minstens 100 mg/dag).
Magnesium supplements are best taken at night to prevent the usual morning deficiency. The dosage of magnesium supplementation depends on the level of its antagonist calcium, since not only magnesium levels are important, but also the serum calcium:magnesium ratio, which should be approximately 2.3:1 for women. This ratio is just as important as the ratio of potassium to sodium. Changes in the Ca:Mg ratio can lead to an increase in the incidence and recurrence of breast cancer *. There is a slight increase in the Ca:Mg ratio in postmenopausal women with cancer (4.9:1) compared to women without cancer (4.4:1) *.
There are several forms of magnesium supplements. Magnesium peroxide, when reduced, forms not only magnesium, but also oxygen, which looks very attractive. However, magnesium ions themselves are poorly absorbed in the intestines. Only a third of the magnesium absorbed through the mouth enters the body. Magnesium taurate, magnesium chloride, and magnesium lactate are more bioavailable than magnesium oxide *. Magnesium glycinate (500 mg/day) or magnesium threonate (2 g/day) are also good magnesium choices. It is also worth considering that of all magnesium consumed in food, only a third is absorbed.
However, it is best to cover your magnesium needs from dietary sources. Rich food sources of magnesium include corn, whole grains, nuts, seeds, legumes, dark green leafy vegetables, seafood, mineralized water, and cocoa *. It's easy to conclude that a vegetable diet is the best dietary choice.
• Iodine (I): up to 3-5 mg, but in no case more than 15 mg/day of iodine.
Iodine is an essential mineral for normal thyroid function and thyroid hormone production *. These hormones control the rate at which carbohydrates are absorbed from food in the intestines and also regulate the rate at which glucose and fat are converted into energy. In addition to the thyroid gland, iodine is taken up by cells in many organs and tissues, including the breast and prostate, maintaining their normal function and integrity * *.
Iodine regulates a number of genes that are involved in estrogen metabolism, cell division, proliferation and differentiation * * *, it also contributes to the detoxification of heavy metals; enhances immune function, prevents recurrence of the disease. In addition, iodine promotes apoptosis of defective cells, inhibiting malignant transformations * * *. In one study, evaluation of urinary iodine in patients with various types of malignancies showed severe deficiency (< 20 μg/L) in 88% of them, moderate deficiency (20-49 μg/L) in 7%, and mild deficiency (50-99 μg/L) in 2% *.
Iodine is an essential element for the growth, development and normalization of female breast tissue; with its deficiency, atypia, dysplasia, as well as benign and malignant tumors of the mammary gland * * are observed. Iodine deficiency increases the sensitivity of breast tissue to estrogen *. It has also been observed that the concentration of iodine in breast cancer tissues is several times lower than in normal tissues or in tissues of benign breast tumors *, and it is not surprising that atypia and malignant tumors in the breast increase local absorption of iodine *.
Iodine inefficiency in «wealthy» countries has increased more than 4 times over the past 40 years, and today 74% of the population here receives insufficient iodine *. Moreover, in US women, compared with men, the average level of iodine is lower, and its deficiency is twice as common *. Although the US Institute of Medicine has set an upper limit for iodine intake of 1'100 μg/day*, this value is likely to be within the norm, not the limit.
Japan is one of the few countries in the world where the dietary intake of iodine is more than adequate *. Japanese consumption of dried algae, mainly Porphyra and Undaria, is ~ 5 g/day, which can provide the body with up to 12.5 mg/day of iodine, not counting its intake from vegetables grown on iodine-rich local soil.
According to various estimates, the average daily intake of iodine in Japan ranges from 5.2 mg to 13.8 mg * *, while in the US it is 216 μg *. However, iodine intake is not the same as absorption; and it seems that the relationship between them is not linear. An analysis of the level of iodine in the urine of the Japanese shows that its actual assimilation on average does not exceed 3-5 mg/day * *. Perhaps, as in the case of vitamin C, the absorption of iodine drops with sufficient concentration in the body.
For the territories, remote from the oceans, iodine deficiency is geographically natural. The lack of iodine in food leads to the fact that in some biochemical processes it is replaced by other halogens (chlorine, bromine and fluorine), which, however, do not perform the biochemical functions of iodine. Chlorination of drinking water exacerbates this imbalance, increasing the relative risk of breast cancer by 18% *. Intense exercise, high sweating and urination *, a vegetarian (and especially vegan) diet *, and several other factors can also contribute to low iodine levels. For example, one study found that a «paleolithic» diet can significantly reduce iodine intake *. High-fat diets are also associated with iodine deficiency *.
Lack of iodine in food impairs the functioning of some organs; first of all – the mammary and thyroid glands, which contain the main reserves of iodine in the body *. Diseases of both these organs often run in sync, and it is significant that cancer in one of them is associated with a risk of cancer in the other *, which may point to common underlying causes, including iodine deficiency.
Iodine deficiency causes an increase in overall estrogen levels *, and a low ratio of estriol to estrone and estradiol *. As you know, both the first and second factors increase the risk of breast, uterine and ovarian cancer. In addition, experiments on rats demonstrate that iodine deficiency increases sensitivity to estradiol and stimulates cell division in the mammary gland *. This effect of iodine deficiency contributes to the development of atypical hyperplasia *, and fibrocystic breast diseases, which affect half of women of childbearing age * and up to 89% of women of advanced age *.
At the same time, molecular iodine (I2) in vitro acts as an antioxidant * and anti-proliferative *, directly promoting apoptosis of various types of breast cancer cells * * * and generally maintaining the health of the female breast *. And in animal studies, iodine has a suppressive effect on the development and size of malignant tumors * * *.
Gamma-linolenic acid (1 g/day from borage oil), iodine (750 μg/day as potassium iodide), and selenium (70 μg/day as sodium selenate) reduces soreness and nodularity in women with fibrocystic disease breast *.
Clinical studies show that higher dosages of iodine may be more successful. Fibrocystic breast disease in women can be reversed * with a long-term daily intake of at least 5 mg of iodine for a year * and a short-term daily intake of 50 mg for 3 months *.
Tumors treated with molecular iodine have less invasive potential and a significant increase in apoptosis, estrogen receptor expression, and immune cell infiltration. Due to this, the relapse-free survival of patients taking iodine supplements (5 mg/day) for 7-35 days before surgery, and then for the entire duration of chemotherapy for 170 days, is doubled compared to placebo *.
Known natural sources of iodine are feijoa, seaweed, Chinese magnolia vine, viburnum, and ocean fish. Combining seaweed with soy improves estrogen metabolism * and significantly reduces the ability of soy to increase serum IGF-1 levels *. Kelp, aka Laminaria has the highest concentration of iodine of any seaweed available.
Seaweed supplements (kelp, porphyry), as well as molecular iodine supplements (0.08 mg/kg ≈ 5 mg/day), in clinical studies have a positive effect on benign tumors *, and significantly (in 65% of patients) reduce the prevalence of breast cysts, fibrous plaques *, and relieve breast pain *. Patients with suspected breast hyperplasia who took 10–20 mg/day of potassium iodide for 6–36 months experienced a significant reduction in pain symptoms, swelling, and nodularity in 72% of cases *.
The level of iodine excretion correlates well with the level of its intake. Since 90% of iodine is excreted in the urine *, measuring urinary iodine concentration can be a reliable method for estimating daily iodine intake *. According to this method, iodine deficiency is defined as a level below 50 μg/L *, and levels of 100-199 μg/L indicate adequate intake *.
However, at such low values, there is no significant relationship between the level of iodine in the body and the incidence in the above graph. It appears that a marked reduction in incidence occurs when iodine intake is increased to 300 μg/day. It can reasonably be assumed that the recommended daily dose of iodine (150 micrograms) helps prevent only the obvious consequences of iodine deficiency, such as goiter, but it is clearly not enough to ensure the health of the female breast.
The degree of iodine deficiency in the Belgian population varies by region, but the mean median urinary iodine concentration in 2019 was 96 µg/L *. Thus, to achieve a relatively safe level (300 μg/day), it is enough to increase the daily intake of seaweed (kelp) – up to 15 g of wet weight, or 2 g of dry weight. Since 1.1 mg/day of iodine is currently considered to be the upper limit of dietary intake *, it is actually possible to triple the intake of kelp.
Women with fibrocystic breast disease may require up to 15 mg/day during treatment; this dosage is close to the regular Japanese diet, and for a short period of time will be safe enough for most Europeans whose diet is poor in iodine *. Women with breast tumors may also be guided by this dosage, which can be achieved by daily intake of 2 drops of 5% Lugol's solution.
Higher doses are unlikely to be of benefit, and instead may stimulate ERα * receptor activity as well as increase the risk of thyroid cancer. Potassium iodide at doses up to 3.6 g/day was prescribed to patients with chronic lung disease for several years *, which was considered acceptable. However, many patients at this dosage developed severe side effects.
Even dietary iodine in chronic excess (> 15 mg/day) can cause thyroid damage *, which has been observed in some Hokkaido residents *. The fact that the population of Japan can consume large amounts of iodine without negative consequences does not mean that the same tolerance for a sharp increase in iodine intake will be shown by the population of other countries who grew up in conditions of its deficiency. Based on this, it will be safer to gradually increase the daily dose of iodine supplements, and at the end of the course of treatment, also gradually reduce it, and then not exceed the maximum threshold.
In any case, when taking supplements, it will be necessary to control the level of iodine in the body in order to avoid both hyper- and hypothyroidism. In addition, people with pre-existing thyroid disease should consult their endocrinologist before increasing their iodine intake, and monitor their thyroid condition more closely while taking it. In addition, for the successful removal of excess iodine from the body, adequate kidney function is required.
Some organs and tissues (such as the breast *, ovaries and prostate) prefer to accumulate molecular iodine (I2), while others (such as the thyroid gland) prefer iodides (I–) *. Lugol's solution contains both of these forms: in a 5% solution there are 50 mg of molecular iodine and 100 mg of potassium iodide per 1 ml (for 20 drops). Therefore, it can be beneficial for the health of many organs, not just the mammary gland. Molecular iodine in equivalent doses can be used to improve the condition of the mammary gland without potassium iodide *.
For iodine to perform its function, certain enzymes (such as iodine peroxidase, which converts iodine to its active form), amino acids (such as tyrosine), and other vitamins (such as B4, B6, and C), and minerals (such as manganese) must work properly. Just as it does with potassium, there are no effective mechanisms for retaining iodine in the body, and therefore iodine is easily lost, excreted in the urine. This circumstance requires a constant influx of new portions of iodine from food.
Despite the importance of iodine, to determine the exact amount of supplements, you should determine its current concentration in the body, and also check it at least 2 times a year, since an excess of iodine can be no less dangerous than its deficiency *. However, such a fear concerns rather pharmaceutical preparations, because it is very difficult to get an excess of iodine with food. The only reliable way to determine the sufficiency of iodine in the body is to measure its level in the urine.
You can get the most rough idea of the sufficiency of iodine in the body with a popular primitive test: using a cotton swab, apply a strip of iodine alcohol solution to a sensitive part of the skin. If traces of iodine disappear before 12 hours, this may be the first sign of iodine deficiency, requiring more accurate confirmation by laboratory tests.
Although other halogens, such as fluorine, are also required by the body in certain quantities, their excess can reduce the biological activity of iodine.
• Selenium (Se): up to 200-400 μg/day of selenium-methyl-L-selenocysteine, L-selenomethionine and sodium selenite.
All three forms of selenium are useful and necessary because they are involved in various biological mechanisms *. Thus, the level of selenium intake can significantly affect both the availability of iodine and the hormonal profile of an individual.
Selenium activates the absorption of iodine; in the physiology of the thyroid gland, both of these elements act together *, and therefore they are recommended to be taken simultaneously. Otherwise, in iodine and selenium deficiency, supplementation of iodine alone can cause irreversible thyroid fibrosis * *, while supplementation of selenium alone causes aggravation of iodine deficiency and hypothyroidism * *.
Selenium reduces the risk of cancer and inhibits carcinogenesis in animals in the early stages of the disease *. Heavy metals, particularly mercury, reduce the bioavailability of selenium, which can cause selenium deficiency. On the other hand, some studies suggest that selenium may have a protective effect against mercury *.
Selenium deficiency increases the risk of cancer. Lower levels of selenium in the blood and tissues are associated with a two- to three-fold increase in the overall risk of developing cancer over the next 5 years * *. Serum selenium concentrations in women with breast cancer are significantly lower than in healthy women *, and the copper:selenium ratio is significantly higher *.
A higher dietary intake of selenium prior to breast cancer diagnosis improves survival and also reduces overall mortality in patients. The group of women with the highest selenium intake, compared with the group of women with the lowest intake, had a 31% lower rate of death from breast cancer *. However, this result may apply just to a population with inadequate total dietary intake of selenium because other studies have not supported the benefit of selenium supplementation for cancer prevention *.
Selenium is involved in the production of antioxidant enzymes such as superoxide dismutase *. This could potentially harm therapies based on high oxidative stress, such as radiation therapy. It can be assumed that during such therapy, its supplementation would be undesirable.
Toxicity of large doses of inorganic selenium has been reported, but this is more likely to be related to selenium supplementation. The intake of selenium from food is not capable of creating its excess, and is not objectionable. Of all natural sources, lemongrass fruit and Brazil nuts hold the record for bioavailable selenium. Two Brazil nuts can contain up to 200 micrograms of selenium, providing a daily requirement. Other natural sources of selenium are garlic, bran, organic eggs, onions, broccoli *.
The reference level of selenium concentration in serum is 130-150 ng/mL; higher and lower rates increase the risk of cancer *. In addition, excessively high doses of selenium contribute to the development of cataracts. Generally, breast cancer patients have significantly lower whole blood and serum selenium levels *, and restoring normal selenium levels could possibly improve their health. However, in each case, a preliminary assessment of the level of selenium in the blood is required before deciding whether to take its supplements *.
Instead of taking one form of selenium, it is recommended to take a mixture of different forms (200 µg each) because each has a different protective effect against cancer, oxidative stress, and DNA damage *.
Selenium-methyl-L-selenocysteine, found in garlic, onions and broccoli, triggers the suicide of cancer cells with a weakened expression of the p53 gene (the so-called «cell suicide gene») *.
Sodium selenite enhances the action of the innate immune system against tumor cells *.
Other forms of selenium act as effective antioxidants, reducing mutagenic transformations leading to cancer *.
The tolerable upper level of selenium intake is defined as 400 µg/day; doses above 2 mg/day are potentially toxic.
• Sulfur (S) stimulates the liver and promotes detoxification. In addition, sulfur is part of the molecule of the main endogenous antioxidant – glutathione, and its deficiency can lead to an increase in oxidative and inflammatory levels. Successful plant sources of sulfur include garlic and cabbage, containing, respectively, disulfide compounds and diindolylmethane. Walnuts, onions, legumes, egg yolks also have a high sulfur content.
• Zinc (Zn): up to 25-50 mg/day zinc oxide; the more accurate dosage depends on the level of copper, since they are antagonists.
Reference ranges for blood zinc levels are 70-120 μg/dL, and values below 70 μg/dL are defined as zinc deficiency *.
Zinc is the second most abundant metal in the body after iron. It is critical for cell growth, development and differentiation *. Zinc regulates about 300 different enzymes and is critical for immune function; however, cancer cells can also use zinc to enhance their defense *. Zinc and magnesium play a key role in estrogen balance and xenoestrogens detoxification.
The body's zinc reserves are typically depleted with increasing age, many illnesses, and high levels of stress. For example, zinc deficiency is common in older people, leading to impaired T-cell function and an increased incidence of respiratory infections *.
In breast cancer, serum zinc levels fall slightly *, although they appear to rise in tumor breast tissue *.
Unfortunately, it would be incorrect to assess the sufficiency of zinc in the body by its concentration in the blood. A low zinc level will indicate that tissue zinc reserves have become so depleted that they are no longer able to provide adequate levels in the blood. Zinc is excreted from the body mainly through feces (90%) and urine (10%). Therefore, it is possible to indirectly assess the adequacy of zinc intake from food by measuring its concentration in urine.
Excess copper can lead to zinc deficiency, and vice versa. Mean serum copper levels in breast cancer were higher than in benign breast disease (167.3 μg/dL vs. 117.6 μg/dL) and in nontumor patients (167.3 μg/dL vs. 98.8 μg/dL). Moreover, the more advanced the disease, the higher the copper level. Compared with healthy women, the Cu:Zn ratio was increased in patients with breast cancer (1.91 vs. 0.86) but not in patients with benign breast tumors * * * *.
On average, middle-aged and elderly women in Europe consume about 10 mg of zinc per day *. This satisfies modern RDA standards, however, during diseases that require intense work of immune cells, this amount of zinc will be clearly insufficient.
Oral zinc supplementation for general zinc deficiency improves immune function and effectively suppresses chronic inflammatory responses *. Divalent zinc supplementation may enhance the antitumor activity of some therapeutic agents, particularly the copper binder disulfiram *. In addition, zinc is a cytoprotector; it protects and stabilizes proteins, DNA, cytoskeleton, organelles, microtubules and membranes *.
Pumpkin seeds are the richest food source of both zinc and magnesium. Other foods rich in zinc include mushrooms, sesame and hemp seeds, seaweed, spinach, Brazil nuts, peanuts, cashews, almonds, pine nuts, wild rice, oats, legumes *. Unfortunately, dietary sources of zinc contain even greater amounts of copper and/or iron. Therefore, to ensure a zinc:copper balance, it is worth considering occasional zinc supplementation, even if it is sufficient in the diet.
The absorption of zinc in the intestine is dependent on the intake of other minerals that use the same transporter, such as calcium, magnesium and iron. The daily absorption limit of this transporter is about 800 mg, and once this is reached, zinc absorption will cease. Thus, you should focus on the total consumption of these minerals in a dosage of up to 800 mg/day. In other words, daily zinc intake should not exceed 50 mg, except for short-term illnesses that require increased intake to enhance immune functions.
In addition, phytates found in some plant foods, as well as iron, copper and manganese, can interfere with the absorption of zinc. This forces you to reconsider your diet towards increasing the consumption of zinc-containing products.
Zinksupplementen kunnen we in verschillende vormen innemen. The absorption capacity of zinc sulfate and zinc acetate is comparable and quite favorable. Zinc picolinate shows increased absorption compared to zinc citrate and zinc gluconate * *. At the same time, zinc oxide and zinc carbonate are poorly soluble in aqueous solutions, resulting in highly undesirable plasma zinc absorption upon oral intake *. There are also many other forms of supplementation that contain varying amounts of elemental zinc *.
Note that in vitro zinc supplementation reduces the susceptibility of cells and tissues to toxin- or radiation-induced apoptosis *, which makes it necessary to be cautious about its supplementation during anticancer therapy.
• Chromium (Cr): up to 50 μg/day.
Trivalent chromium helps insulin deal with spikes in blood glucose levels, reduces blood fat levels, and also makes it difficult for cancer cells to get glucose. In addition, chromium ensures the efficient functioning of the immune system. Chromium deficiency is difficult to detect due to the lack of accurate tests. Trivalent chromium, unlike hexavalent chromium, is non-toxic and has not been established an upper limit of intake. At the same time, divalent chromium stimulates the proliferation of breast cells through the estrogen receptor ER-α *.
Good natural sources of chromium are brewer's yeast and Sea buckthorn (Hippophae). Enough 1 tbsp. sea buckthorn to provide a daily allowance of chromium. If there is a question of taking supplements, then 400-1'000 mg/day of chromium picolinate is recommended.
• Molybdenum (Mo): up to 50-100 μg/day of chelated molybdenum.
Molybdenum, like zinc, is a physiological copper antagonist. Consumption of molybdenum in drinking water at a concentration of 10 mg/L significantly inhibits the formation and growth of carcinogen-induced mammary tumors in rats * *.
Copper depletion with the copper chelator tetrathiomolybdate (100 mg/day) results in a reduction in cancer stem cells in patients with TNBC *.
A phase II clinical trial showed that tetrathiomolybdate (100 mg/day for 2 years) was able to prevent recurrence in patients at high risk of breast cancer recurrence *.
The combination of tetrathiomolybdate with doxorubicin significantly slows down the growth of an inflammatory subtype breast tumor *.
Plant sources of molybdenum are viburnum fruits, legumes, grain bran, nuts. Unfortunately, it is not possible to achieve copper:molybdenum balance through dietary modification, so supplementation of 50-100 μg/day of tetrathiomolybdate is worth considering. The Tolerable Upper Intake Level for molybdenum has been set at 2 mg/day.
• Boron (B). The daily intake of boron has not been established, but it should not exceed 3 mg/day.
Boron improves the absorption of magnesium and calcium and reduces their loss, supporting bone tissue; increases the level of antioxidant enzymes; accelerates wound healing; beneficial effect on the metabolism of estrogen, testosterone and vitamin D; normalizes the functioning of the thyroid gland; reduces levels of inflammatory biomarkers; improves brain function; increases resistance against breast and prostate cancer and reduces the side effects of radiation and chemotherapy *.
The main source of boron, like most of the other elements discussed here, is plant foods. The richest in boron are avocados and dried fruits (raisins, dried apricots and prunes).
• Germanium (Ge): up to 100 mg/day sesquioxide.
Germanium is able to increase the level of interferon, macrophages, T-suppressor cells, and enhance the activity of natural killer cells *. Examples of natural sources of germanium include garlic, ginseng roots, and dandelion.
• Cesium (Cs): up to 2 mg/day cesium chloride.
Increasing the saturation of the body with cesium greatly reduces the risk of breast cancer, regardless of its subtype *. However, high doses of cesium appear to be able to reduce acidity within cancer cells, which may be incompatible with therapy aimed at increasing intracellular acidity.
• Silicon (Si) contributes to the integrity of the connective tissue, which makes it difficult for cancer to invade and metastasize.
• Lithium (Li): up to 300-1'000 μg/day of lithium orotate.
The longevity of people is directly associated with the content of lithium ions in drinking water * *.
Lithium has been shown in preclinical studies to help maintain longer DNA telomeres, regulate genes associated with healthy DNA and their structure, and protect cells from aging. In Alzheimer's disease, lithium supplementation has been shown to reduce symptoms of the disease *.
The risk of cancer, especially breast cancer, is increased in women with bipolar disorder, and lithium may be of interest as a protective measure in both cases *. Lithium salts under certain conditions also exhibit anti-inflammatory properties *, and in vitro can enhance the effect of such an antitumor drug as mitomycin C *. However, there is no clinical evidence of its benefit in breast cancer.
Like most other similar cases, the benefits of taking lithium compounds seem to manifest themselves only when the deficiency of lithium is eliminated, and not when it is in excess. However, an adequate level of lithium in the body has not been established.
• Nitrogen (N) in the form of nitric oxide relaxes and dilates blood vessels, improving oxygen delivery. However, not all dietary sources of nitrogen are equal. Nitrates (found in arugula, spinach, red beets) help reduce the risk of stomach cancer, while nitrites are carcinogenic and increase the risk of thyroid cancer and glioma *.
At the same time, the intake of some other chemical elements can play, on the contrary, in favor of the tumor.
• Iron (Fe) is an important element that is involved in oxygen transport and tissue oxygenation, electron transport, energy metabolism and DNA synthesis. However, excess free iron can catalyze chemical reactions that produce free radicals, which cause oxidative stress and cause damage to protein structures.
From a nutritional point of view, there are two types of iron – heme and non-heme. Heme iron is found exclusively in meat, poultry and fish as it is a component of hemoglobin and myoglobin. Dairy products and eggs do not contain heme iron. Non-heme iron is present in both plant and animal foods; for example, meat contains about equal amounts of heme and non-heme iron (red meat has a worse ratio than white). Cooking meat only slightly reduces heme iron levels from baseline *.
Heme iron, even in high-meat diets, is < 10-15% of iron intake. But due to the fact that heme iron is absorbed better than non-heme iron, it can account for up to 30% of all iron received from food. Humans do not have effective mechanisms to regulate iron levels in the body. Therefore, high consumption of meat products can lead to excess iron in the body. Food sources richest in heme iron are clams, oysters, mussels, and animal livers.
Non-heme iron makes up ~ 90% of the iron in most diets. Rich dietary sources of non-heme iron include green leafy vegetables, dried fruits, molasses, nuts and seeds, and fortified cereals. Supplements taken for iron deficiency are the non-heme form.
Making a responsible choice to limit or increase iron intake depends on the need identified as a result of the relevant research. Iron testing is one of the most important routine tests for patients because its safety range is very narrow and the consequences of going outside that range are very dramatic.
On the one hand, excessive iron deficiency can lead to anemia and a decline in the immune system, which is observed in a quarter of the world's population *. On the other hand, even a slight excess of iron promotes inflammation as well as cell division and thus enhances tumor growth * *. Increased levels of ferritin (a protein that stores and transports iron) is associated with a significant increase in the risk of fibrocystic conditions, with an increase in breast cancer *, with a risk of death from all causes *, and also with a poor prognosis in metastatic breast cancer *.
Reactive oxygen species produced during normal aerobic cellular metabolism can lead to the release of free iron from ferritin. In the presence of superoxide radical and hydrogen peroxide, stored ferric iron (Fe3+) is reduced to ferrous iron (Fe2+). The hydroxyl radical ·OH produced by this reaction may promote lipid peroxidation, mutagenesis, DNA strand breaks, activation of oncogenes, and inhibition of tumor suppressors, increasing the risk of breast cancer *.
The concentration of ferritin in the tissues of malignant breast tumors is several times higher than in benign tissues *. If in healthy women the average level of ferritin in serum was 45.5 ng/mL, then in women with a benign tumor – 48.4 ng/mL, and in women with a malignant tumor – 81 ng/mL *. A particularly significant increase in ferritin levels was observed in PR-negative tumor subtypes. In this regard, the level of ferritin in the blood should be in the range of 15-45 ng/mL * *.
In cancer, the level of iron in the breast tissue is slightly increased * *, while iron deficiency in other tissues is a typical picture. High levels of iron intake in observational studies have been associated with increased tumor growth * * – each additional 1 mg/day iron intake increases the risk by 8% *. Conversely, a diet low in iron can reduce the risk of cancer * and lead to slower growth of existing tumors * *. Finally, taking iron supplements after a cancer diagnosis is associated with an increased risk of death by up to 39% *.
Thus, overloading the body with iron may contribute to the development of cancer. For this reason, the consumption of red meat (more than 9 g/day), and even more so, the consumption of animal blood, can definitely be considered a conditionally carcinogenic factor * *, especially since WHO classifies meat as a group 1 carcinogen *. Every 1 mg of iron from animal foods increases the risk of cardiovascular disease by 5% *, which means eating just one hamburger a day increases the risk by 10%.
Antioxidants can somewhat weaken the carcinogenic effect of dietary iron *, while lipids, on the contrary, increase it *. Therefore, it seems reasonable to recommend consuming animal protein along with plenty of greens. Limit the intake of iron calcium, magnesium, garlic, vitamin E, green tea, dry red wine, flax, as well as dietary fiber, which contains phytic acid. Phytonutrients that can bind and remove excess free iron from the body include curcumin, EGCG, proanthocyanidins, ferulic acid, baicalin, quercetin *.
Phytic acid (IP6) is also able to chelate iron, reducing iron-induced oxidative stress *. Therefore, IP6, like other iron chelators, is undesirable in artemisinin and other therapies that use oxidative stress.
The body is reluctant to part with iron, so its levels can gradually increase, reaching undesirable values. High levels of iron in the body are observed in postmenopausal women. Blood donation can reduce the danger of iron, however, on the condition that after this procedure, the intake of iron will again not significantly exceed its losses.
• Copper (Cu) is necessary for the normal functioning of the body. It is involved in the work of many enzymes; required for the development and functioning of the nervous and cardiovascular systems and fundamentally important for the hormonal, reproductive and immune systems. However, at elevated concentrations, copper becomes toxic and, like iron, causes oxidative stress.
Breast cancer tissues, compared to healthy tissue, demonstrate an increase in copper concentration by 1.5-2.5 times * *. The serum Cu:Zn ratio also increases with the development of a malignant but not benign tumor *.
High serum copper levels (27 μM) versus low levels (6 μM) are associated with a 50% increase in the relative risk of all-cause mortality, a 40% increase in cancer mortality, and a 30% increase in cardiovascular mortality *. Excess copper stimulates metastasis and tumor angiogenesis * *, while copper removal by a chelator can significantly suppress in vivo angiogenesis (SUM149) * as well as markers typical of EMT (MCF-7) *.
Thus, inappropriate use of copper supplements may contribute to tumor development. Given that more than adequate amounts of copper are usually supplied through the diet, copper supplements cannot be recommended. An exception is when copper is deliberately introduced to increase oxidative stress during chemotherapy, and the excess is removed at the end of therapy.
Natural sources of copper include sesame seeds, Brazil nuts, hazelnuts, cashews, pine nuts, cocoa, legumes *.
The most famous copper chelators are tetrathiomolybdate, disulfiram (antabuse), curcumin, bleomycin, ionophores.
• Heavy and toxic metals show pathological accumulation in breast tissue and are suspected of being directly related to the process of malignant growth * *. Many metal ions can create oxidative stress as well as bind to estrogen receptors and thus exhibit estrogenic effects *. These are, in particular, aluminum, antimony, arsenic, barium, chromium, nickel, cadmium, cobalt, copper, tin, lead, mercury and vanadium.
Arsenic, for example, is classified as a Class I * carcinogen, along with asbestos, cigarette smoke, formaldehyde, plutonium, and processed meats. Lead can replace iron or calcium in molecules, taking their place; but cannot replace iron or calcium in the performance of their functions. For example, replacing iron with lead in a hemoglobin molecule deprives it of its ability to carry oxygen. Cadmium can replace zinc, thereby impairing immune function. And replacing calcium with lead in brain cells deprives them of their ability to process messages from nerve cells.
Some plant and food products have weak properties to bind and remove heavy metals from the body. These are Garlic (Allium sativum), bulb; Milk thistle (Silybum marianum), seeds; Coriander (Coriandrum sativum), leaves; Ginkgo biloba (Ginkgo biloba), leaves; Turmeric (Curcuma longa), root; Amla (Emblica officinalis), fruits; soluble vegetable fiber such as pectin; as well as green algae such as Chlorellales *. And although their effectiveness is much lower than specialized chemicals, their long-term systematic use can tip the balance of debit and credit of toxic metals in a favorable direction for us.
Even a slight change in the level of trace elements in the tissue causes a violation of its metabolism. As in the case of vitamins, an excess of many chemical elements can be no less harmful than their deficiency *. The same can be said about their imbalance. Therefore, the basis for making a decision on taking supplements of elements, as well as on their dosage, can only be the results of regularly conducted biochemical analyzes.
Another important factor in favor of correcting the level of ions of a particular element may be the therapeutic status of the patient. High levels of iron and copper increase the concentration of reactive oxygen species. Thus, in the post-treatment period, reducing copper and iron levels may be beneficial, while during radiotherapy and chemotherapy using high oxidative stress, on the contrary, their supplementation may be useful. But with zinc, the situation seems to be just the opposite.
Balance of elements. As in many other cases, not only the quantitative content of elements, but also their balance plays an important role.
So, the optimal ratio of Ca:Mg in plasma should be 2:1, but not lower than 1.7:1 and not higher than 2.8:1. A high dietary Ca:Mg ratio (> 2.59) is associated with lower all-cause mortality, especially for postmenopausal women *.
The optimal ratio of Cu:Zn in plasma should be in the range of 0.7-0.8:1 * *. The predominance of copper over zinc is a very unfavorable factor in the development of cancer *. A low zinc to copper ratio increases oxidative stress *. With age, the ratio tilts in favor of copper, and worsens the prognosis of all-cause mortality *.
The ratio of copper to zinc in breast cancer tissue increases not only in comparison with normal tissue, but even in comparison with benign tumor tissue *, and increases as the disease progresses *, that is, it is associated with a malignancy indicator. Chemotherapy is able to further increase copper predominance by lowering zinc levels *, suggesting a benefit from zinc supplementation after anticancer therapy.
Cancer patients also show an increase in the plasma copper:selenium ratio (2.1:1) compared to healthy women (0.9:1) *.
A low dietary Ca:P ratio (~ 1:2) appears to also significantly increase the risk of breast cancer *.
Measurements of blood plasma parameters in various studies show that in patients with breast cancer, the levels of copper, manganese and molybdenum are significantly higher than in healthy people, while the levels of selenium, on the contrary, are lower * *.
Mineral level control. To make a decision about the need for mineral correction, it is required to find out the current level of the elements of interest. However, the exact determination of the physiological excess or deficiency of one or another element presents certain difficulties. The composition of the blood is most strictly controlled in the body; with a deficiency of one or another component in the blood, it is extracted from the tissues, and with an excess, it is deposited in the tissues. For this reason, blood tests in many cases will not say anything definite about the systemic deficiency of the most important chemical elements; to present an objective picture, a chemical analysis of the tissues of interest to us will be required.
It is impossible, for example, to clearly assess calcium or phosphorus deficiency without a bone mineral analysis. However, a blood test can tell us quite definitely about the levels of elements such as zinc, copper, magnesium, selenium, cadmium, lead, and mercury *.
Urinalysis can tell us about the levels of elements such as iodine, bromine, selenium, arsenic, cadmium and mercury. Strictly speaking, urinalysis displays what is excreted and not what is present, however, in many cases it gives an idea of the level of these elements in the body.
Hair analysis can also provide some insight into the mineral composition of the body. Although hair and nails can be significantly contaminated by external influences, their analysis can suggest an excess of toxic metals (such as lead, mercury, arsenic and cadmium), as well as determine the approximate levels in the body of essential minerals – calcium, magnesium and selenium.
The Academy of Nutrition and Dietetics of the USA takes the position that vitamin and micronutrient supplements are justified if their adequate dietary intake is not met. People with chronic diseases, those who are taking medications, those who are pregnant and breastfeeding, those who are growing rapidly, and those of advanced age are especially at risk. At the same time, the regular and indiscriminate use of micronutrient supplements to prevent chronic disease is not recommended due to the lack of scientific evidence *.
From all of the above, the following conclusions can be drawn:
- the main source of vitamins and chemical elements should be food;
- following the suggested dietary recommendations may eliminate the need for supplementation;
- the need to take certain additives should be justified by their deficiency, which is detected by laboratory means;
- supplements should make up for the deficiency, and not significantly exceed the physiological norm;
- vitamin D, iodine, selenium, zinc, magnesium and calcium deficiencies are the most common in European coutries, and it can be assumed that the periodic intake of these supplements in small doses will bring more benefit than harm.
It should also be remembered that the average daily requirements for vitamins and minerals discussed above only reflect the norms that satisfy the majority, but the entire population. Each person has individual characteristics of what should be considered «normal» consumption. But unfortunately, we do not yet have the ability to measure or evaluate this «normality» otherwise than relying on a person's inner intuition, expressed in his food desires.
In addition to vitamins, minerals and essential amino acids, there are other essential substances, without which the human metabolism will not be complete, which can negatively affect overall health. These are, for example, essential carbohydrates, essential amino acids and essential fatty acids.
Essential amino acids for a healthy adult include valine, isoleucine, leucine, lysine, methionine, threonine, tryptophan, and phenylalanine. In addition, histidine is an essential amino acid for children. A balanced diet can fully satisfy the need for them. A possible imbalance in the intake of essential amino acids can be easily eliminated by using tables of the content of essential amino acids in various foods. For this, the following proportions are guided: tryptophan – 1 part, threonine – 2 parts, phenylalanine – 2 parts, methionine – 3 parts, lysine – 3 parts, valine – 3 parts, isoleucine – 3 parts, leucine – 3.5 parts.
Additional intake of essential amino acids for breast health is not required, rather the opposite * *. The essentiality of amino acids does not mean that they should be consumed in high quantities. It is known that, in addition to increased glycolysis, cancer cells differ from normal ones in the uptake and production of certain amino acids *, which ensure the production of the main part of carbon- * and nitrogen-containing * biomass for rapidly proliferating cancer cells. Thus, a generous supply of amino acids from dietary protein may promote tumor development. And vice versa, limiting the raw material base for building new cells will, rather, contribute to the inhibition of tumor growth.
In addition to the general restriction of amino acid intake, it is also possible to target the restriction of a particular amino acid, since cancer cells are more sensitive to deficiency of some of them than normal cells. An example is methionine *. In addition to its antitumor effect, dietary restriction of methionine has the same longevity-prolonging effect as general dietary restriction *. The content of methionine in vegetable protein is 2-3 times lower than in animal protein.
Essential fatty acids are a group of polyunsaturated fatty acids that cannot be produced by the human body. In fact, there are only two essential fatty acids (EFAs) from which other fats can be synthesized. These are linoleic acid (LA) – a precursor of ω-6 series fatty acids, and alpha-linolenic acid (ALA) – a precursor of ω-3 series fatty acids. For example, ω-3 fatty acids such as eicosapentaenoic (EPA) and docosahexaenoic (DHA) are easily obtained from fish oil.
Polyunsaturated fat (PUFA) intake > 10% of total energy intake in women was associated with a 2.5-fold reduction in breast cancer risk compared to intake < 10% *. Every 100 mg/day of ω-3 in the diet provides a 5% reduction in the risk of breast cancer *. In this case, however, it is worth observing the measure, since a high consumption of fats, especially saturated fats, increases the incidence of breast cancer *. Prospective studies have also firmly established the protective role of ω-3 fatty acid intake from fish oil * *.
Interestingly, the source of EFAs may be relevant to the risk of morbidity. Intake of ALA (ω-3) directly from fruits, vegetables, and vegetable oils is associated with a reduced risk, while its intake from mixed nuts and processed foods is associated with an increased risk *. Apparently, other food components are able to enhance or weaken the positive effect of ω-3 fatty acids.
In addition to absolute values, the ratio of fatty acids consumed is important *. In rats, a high ratio of ω-3:ω-6 (1:1) produced lower levels of inflammatory markers and a better reduction in obesity than a low ratio (< 1:4) *.
In most human studies, women with a high ratio of ω-3:ω-6 fatty acids consumed show a lower risk of developing breast cancer compared to women with a low ratio * * * * * * *. In particular, a low (< 0.2) ω-3:ω-6 ratio is associated with a two-fold increased risk of breast cancer compared to controls * *. The ideal ratio ω-3:ω-6 seems to be 1:2-1:2.5 * *; in any case, it should not go beyond 1:5 * *. Fatty acids ω-6 are consumed in the modern diet more than enough, shifting the balance of fatty acids consumed in an unfavorable direction. In Belgium, for example, the average ratio ω-3:ω-6 exceeds 1:30.
The mechanisms of action of ω-3 include the reduction of pro-inflammatory lipid derivatives, the inhibition of cytokine production, and the reduction of growth factor receptor signaling as a result of changes in the lipid layer of the cell membrane. ALA supplementation (15 ml of flaxseed oil, i.e. 1 tablespoon per day) for 3 months significantly reduced the levels of C-reactive protein (by 38%), serum amyloid A (by 23%) and IL- 6 (by 10%) compared to the original values *.
The generally recommended intake of a mixture of EPA and DHA is 250-500 mg/day *, which corresponds to approximately 1-1.5 g of fish oil; permissible upper limit – 5 g/day *.
Essential carbohydrates (glyconutrients) * are a recently announced class of essential carbohydrate nutrients, along with essential amino acids and essential fatty acids. Previously, carbohydrates were considered solely as a source of energy, but now they are also considered as participants in the transmission of cellular signals. By forming glycoproteins, carbohydrates provide a much greater number of combinations of the structure of signaling molecules than amino acids alone can provide.
Some simple carbohydrates are essential for the production of properly structured and therefore properly functioning glycoproteins involved in cell communication. Although this idea has not been convincingly proven, it is assumed that their deficiency leads to a change in the structure of glycoproteins, and therefore to disruption of cellular communication, which can contribute to a number of degenerative diseases, including cancer.
The modern Western diet has a monstrous imbalance of simple carbohydrates in favor of sucrose (sugar), which is metabolized to fructose and glucose. Galactose is also usually consumed in sufficient quantities, which cannot be said for other simple sugars. The food of Paleolithic man was more varied, including in relation to simple sugars, as well as mucus and gums, for example, the resin of fruit trees.
Eleven main biologically active simple sugars have been identified – glucose, D-mannose, L-fucose, D-xylose, D-galactose, L-arabinose, N-acetylglucosamine, N-acetylgalactosamine, N-acetyl-neuraminic acid, L-iduronic acid and glucuronic acid. Some of them usually cannot be obtained in sufficient quantities from the food taken. Dietary adjustments or, in extreme cases, some supplements could correct the imbalance in the intake of simple sugars. These are, for example, supplements such as aloe vera (mannose); fenugreek seed (mannose, galactose); kelp (fucose, xylose, mannose, galactose); medicinal mushrooms (fucose); brewer's yeast (fucose); whey protein concentrate (N-acetylneuraminic acid); animal cartilage (N-acetylglucosamine, N-acetylgalactosamine); psyllium seed (xylose).
Some nutritional supplements contain ready-made complexes of all 11 bioactive sugars, combining such natural sources as guar (E412), acacia, arabic, xanthan or dextran gums; larch (E409), cherry, plum, carob, karaya gums; raw sugar; agar; algin; astragalus; gummi gatti; pectin; chitin; hemicellulose; inulin and others *. That is, those food products that do not get into food because of the inferiority and monotony of the modern urban diet. One of the supplements offers the following composition of #00 capsules: galactose – 25 mg, fucose – 50 mg, mannose – 110 mg, xylose – 38 mg, acetylglucosamine – 38 mg, acetylneuraminic acid (sialic acid) – 38 mg, acetylgalactosamine – 50 mg. Supplementation with 2 of these capsules per day can provide significant antiviral and antifungal support to the immune system *.
Essential carbohydrates do not accumulate in the body, that is, they do not create their own reserve. Depending on their chemical structure, they are metabolized within minutes to hours. Thus, for their constant concentration, they require regular intake. In contrast to essential carbohydrates, simple sugars (such as sialic acid) can promote the production of glycoproteins that shield cancer cells from immune surveillance and thus promote metastasis *. In prostate cancer, high levels of sialic acid in the tumor are associated with a poor prognosis, while high levels of fucose are associated with a good prognosis *.
The most accessible source of essential carbohydrates is the resin of the apricot tree. It is two-thirds carbohydrate, and is rich in L-arabinose, D-galactose, xylose, mannose and rhamnose, respectively, 41%, 24%, 18%, 14% and 3% of all monosaccharides (in mole percent) *. It can be collected throughout the summer. Cherries, plums and other stone fruits are less prolific for resin. Interestingly, in Iran, apricot resin is used in folk medicine for coughs, as well as to improve complexion. Stone fruit gum can also be used as a natural pharmaceutical excipient that increases the bioavailability of nutraceuticals, slows their absorption (and therefore metabolism), and improves their delivery within the body.
Antioxidants can reduce oxidative damage to cell structures, reduce inflammation, inhibit proliferation and angiogenesis, and enhance apoptosis *. In addition, they are able to mitigate the toxicity of standard cancer treatments by protecting normal cells *.
The preventive potential of antioxidants has been studied in at least two large-scale clinical trials. One placebo-controlled study was conducted in China and lasted about 7 years. Here, patients in 4 different groups were given supplements: retinol (5'000 IU/day) and zinc (22.5 mg/day); riboflavin (3.2 mg/day) and niacin (40 mg/day); vitamin C (120 mg/day) and molybdenum (30 μg/day); and beta-carotene (15 mg/day), vitamin E (30 mg/day) and selenium (50 μg/day). Daily dosages of supplements were 1-2 times higher than those recommended in the USA.
The most dramatic reductions in morbidity (16%) and mortality (10%) from esophageal cancer were seen in those who received beta-carotene, vitamin E, and selenium supplements *. The positive effect of supplementation could be a result of the fact that the initial level of micronutrients in the population was very low for dietary reasons. Interestingly, the risk reduction did not begin to appear until 1-2 years after the start of supplementation.
Another similar 7-year clinical trial (SU.VI.MAX) conducted in France also tested the effectiveness of antioxidant supplements in reducing cancer incidence. The general list of supplements included vitamins C (120 mg/day), E (30 mg/day) and beta-carotene (6 mg/day), as well as minerals – selenium (100 μg/day) and zinc (20 mg/day) *. Here, a 31% reduction in overall cancer incidence and a 37% reduction in overall mortality was seen in men, but not in women, who had higher baseline levels of vitamins and minerals compared to men *.
Based on this, we can conclude that the preventive effectiveness of antioxidants is manifested only when their deficiency is compensated, but not when they are in excess.
Once diagnosed, taking antioxidant supplements, if deficient, may also be helpful. It seems that the decrease in the total antioxidant level goes in parallel with the development of the tumor process.
Meta-analyses show that vitamin C intake (100 mg/day) after cancer diagnosis reduces breast cancer mortality by 22% and overall mortality by 22% compared to population averages *. Women who took moderate doses of supplements (vitamins A, B, C, D, E, multivitamins) within 6 months of being diagnosed with invasive breast cancer had an 18% lower risk of mortality and a 22% lower risk of recurrence *. Feedback was also observed in women who underwent surgery, regardless of whether vitamins were taken concomitantly with chemotherapy or not, however, it was clearly expressed only among those patients who did not receive radiation therapy.
Evaluation of post-treatment supplementation (vitamins A, B, C, D, E, and multivitamins), 1-5 years after diagnosis, found that multivitamins, vitamins C, or E were not associated with relapse but were associated with a reduced risk death by 16%. In addition, vitamin D has been associated with a reduced risk of recurrence among ER-positive but not ER-negative tumors *.
During therapy, antioxidants can support not only healthy cells, but also cancer cells, and we have no reliable way to selectively deliver them to exclusively healthy cells. This causes a natural debate about the appropriateness of antioxidant supplements during this period.
The prevailing view is that antioxidants can interfere with primary treatment because both radiation therapy and many chemotherapy drugs use oxidizing agents to damage the DNA and cellular organelles of cancer cells. Guided by the «Do No Harm» principle, doctors do not recommend taking any drugs that are not related to the main treatment protocol during anticancer therapy. However, there is strong evidence to suggest that, in most cases, the benefits of antioxidant support for healthy cells may outweigh the harms of supporting cancer cells.
Although it is generally accepted that cellular damage caused by some chemotherapy drugs is due to oxidation, most of them kill tumor cells by interfering with DNA metabolism through non-oxidative mechanisms. Based on this, it can be assumed that antioxidants can prevent unwanted tissue damage without affecting the effectiveness of chemotherapy *. Moreover, oxidative stress associated with inflammation in tumor tissue can make it difficult to kill the tumor. The reason for this is that oxidants can interfere with apoptosis caused by chemotherapeutic agents (etoposide, doxorubicin, fluorouracil), but this can be overcome with the help of antioxidants * *.
Indeed, an analysis of 50 clinical trial reports involving 8'521 patients showed that over-the-counter antioxidants and some other supplements do not reduce the effectiveness of radiation/chemotherapy for cancer. On the contrary, they increase the damaging effect of therapeutic agents, reduce their side effects, and protect normal tissue. Beta-carotene was considered here; vitamins A, C, E, D3, К3; group B vitamins; selenium; cysteine and glutathione. Patients who took antioxidants and other nutrients actually had an increased survival * *.
Another review of 19 clinical trials found no evidence of a significant reduction in efficacy from antioxidant supplements during chemotherapy. Some studies have shown no benefit from antioxidant supplements. However, in many others, antioxidant supplementation has been shown to increase survival time, enhance therapeutic response, and reduce chemotherapy toxicity compared to controls *. Here, the list of antioxidants included glutathione, melatonin, vitamin A, vitamin C, N-acetylcysteine, vitamin E, and ellagic acid.
Finally, a 2018 review collected results from thirteen different antioxidants and their analogues, either alone or in combination with chemotherapy. This included 174 peer-reviewed original articles from 1967 to 2017, including 93 clinical trials with a total of 18'208 patients. The data confirmed that antioxidants have enormous potential to reduce chemotherapy-induced toxicity. In addition, antioxidant supplementation during chemotherapy predicted higher therapeutic efficacy and longer life expectancy for patients *.
Another 2021 meta-analysis found that the use of antioxidant-vitamin supplements after diagnosis did not worsen breast cancer survival, while the use of vitamin C after diagnosis could markedly improve overall survival *.
In addition to the previously discussed vitamin antioxidants, other antioxidant substances can be used.
• Methoxanthin, also known as pyrroloquinoline quinone (PQQ) and vitamin B14, is a powerful water-soluble antioxidant. Methoxanthin is a coenzyme critical for cellular energy homeostasis and redox balance *. Methoxanthin protects cells during mitochondrial stress and increased oxidative load *.
A methoxanthin-supplemented diet provides greater energy expenditure by increasing the number of mitochondria in liver tissue, and also protects the heart from lack of oxygen *, which can protect heart cells in a heart attack. Consumption of 25 mg/day of methoxanthin enhances anti-inflammatory potential by reducing plasma levels of IL-6 and C-reactive protein *. In addition, as early as 20 mg/day of methoxanthine disodium for 12 weeks normalizes low-density lipoprotein levels in patients *, increases cerebral blood flow, and also helps prevent deterioration in brain function in the elderly, especially with regard to attention and working memory *.
• Coenzyme Q10 (CoQ10) is a fat-soluble antioxidant, immune system stimulant, mitochondrial respiration enhancer. CoQ10 controls the expression of several hundred genes, many of which are involved in inflammatory signaling, in particular NF-κB *.
As an adjunct to the main treatment, and in combination with vitamins C, E, beta-carotene, pyroxidal phosphate, selenium and ω-3 fatty acids, CoQ10 has been shown to increase the survival of breast cancer patients and even tumor regression * *. Low levels of circulating CoQ10 have been associated with a high risk of breast cancer, as have levels that are too high; and circulating CoQ10 levels in the range of 500-800 ng/mL had the lowest risk of developing breast cancer *. Dosage: 100-400 mg/day.
• Alpha lipoic acid (thioctic acid). Vitamin-like antioxidant substance, the main antioxidant in cellular mitochondria. Enhances the action of other antioxidants and vitamins. Helps reduce blood glucose concentration and general detoxification. Dosage: 400-1'000 mg/day.
• Melatonin. A nighttime hormone that declines as we age. Protects body cells from oxidative damage at night. Take 3 mg at bedtime.
• Epigallocatechin gallate (EGCG). A strong water-soluble antioxidant. Reduces the activity of the growth factor VEGF, activates lymphocytes, inhibits angiogenesis and metastasis in the initial stages of the disease. Dosage: 10 g/day decaffeinated green tea dry extract (600-1'000 mg/day pure EGCG). Take 3 months, then a break of at least 3 months.
Pure EGCG may be preferred as green tea, in addition to its benefits, is able to increase dihydrotestosterone levels.
Alternatives: green tea catechins in the form of dietary supplement Tegreen™, green tea «matcha».
It is worth recognizing, however, that not all studies support the benefit of antioxidant supplements during chemotherapy. Although in smaller numbers, there are contradictory data, and such uncertainty requires special consideration.
The reasons for the ambiguity of the results of various studies, among other things, can be explained by the different conditions of action of antioxidants.
The first graph shows that with a low saturation of cells and tissues with free radicals, the latter do not have a significant negative effect. However, as oxidative stress increases, they begin to act proliferatively, stimulating cell growth. Finally, at very high concentrations, free radicals destroy proteins and lipids in both cancerous and healthy cells, causing their death.
Under different initial conditions, the intake of additional antioxidants can lead to directly opposite results. Let's consider on the next graph the ratios of all three factors – antioxidant reserves in the body, ROS concentration and cell proliferation. Let's single out with a separate color patients with initial relatively low, medium or high levels of antioxidant reserves (orange, green and purple, respectively).
It becomes clear that as the concentration of free radicals increases, the antioxidant reserves of the cancer cell decrease for all three groups of patients. Moreover, cancer cells in patients with low antioxidant reserves are more susceptible to oxidative stress than in patients with high reserves. For example, the G-point corresponding to the cessation of proliferation is more likely to occur in patients with a low supply of antioxidants (orange) than in patients with a high supply (purple).
Now let's try to evaluate the consequences of taking antioxidant supplements for various specific situations.
• The situation of moderate oxidative stress with an average supply of antioxidants in the cancer cell (point D on the green line).
Such a case may be in a patient who has not undergone radiation or chemotherapy, due to which the concentration of ROS is not very high. If, at a constant ROS level, the patient is taking a dose of antioxidants sufficient to create high reserves, then we should draw an imaginary vertical line upward from point D to the purple curve (curve of the patient with high antioxidant reserves), where we will get to the place between points C and D. Referring now to the previous graph, we see that moving from point B to a place between points C and D will cause a slight increase in proliferation.
• A situation of moderate oxidative stress with a low supply of antioxidants (point D on the orange line).
Such a case is possible in a patient who has not undergone chemotherapy. If the patient begins to raise the level of antioxidant reserves to a high level, then we should draw an imaginary line from the orange point D up to the purple line, where we will fall between points B and C. Referring again to the previous graph, we see that the movement from point D to the place between points B and C causes a marked decrease in proliferation due to a decrease in oxidative stress, i.e. by reducing inflammatory signals.
If we take point E instead of D for consideration, then an increase in the level of antioxidants will produce a movement from point E to a place between points C and D, which will practically not affect proliferation.
• The situation of moderate-high oxidative stress with a low supply of antioxidants (point F on the orange line).
In a terminal patient who has not undergone chemotherapy, antioxidant reserves may be depleted, and oxidative stress may be high, but not as high as it would be with chemotherapy. Proliferation, judging by the upper graph, is constrained by a high oxidative level. The increase in antioxidant stock brings us to a place between points D and E on the purple curve. The top graph shows that moving from point F to points D and E can cause a modest increase in proliferation.
• A situation of high oxidative stress with a low supply of antioxidants (point H on the orange line).
The increase in antioxidant stores in this case brings us to point F on the purple curve. The upper graph shows that the position for cancer cells fundamentally changes: they move from the zone of death to the zone of moderate proliferation. It becomes clear why low (oral) doses of vitamin C, instead of killing the tumor, can sometimes cause a significant increase in proliferation.
This case study by John Boik * explains why the effects of increasing antioxidant levels may differ depending on initial conditions. None of the numerous clinical studies have measured the initial antioxidant capacity of cells. The large scatter of the obtained results caused by this did not allow an objective assessment of the antitumor role of antioxidant supplements, but it led to conflicting conclusions.
These graphs make clear the complex role of antioxidants. During the non-therapeutic period, antioxidants reduce oxidative stress, thereby reducing inflammation and cell proliferation. During the therapeutic period, antioxidants may protect cancer cells. However, in the latter case, they can also stimulate proliferation, which introduces cancer cells into the division cycle, thereby exposing them to the lethal effects of chemotherapeutic cytotoxins.
Given the heterogeneity of the tumor, antioxidant supplements can affect different parts of the tumor in different ways. In some places, proliferation will be reduced due to the weakening of oxidative stress; in others it will not change; and in the third it will be increased, which can enhance the therapeutic effect of the standard protocol. Given the protective role of antioxidants for healthy cells, it becomes clear why they have been found to be beneficial in most of the studies reviewed above, although not all.
Antioxidants may also have biological effects on cancer cells that are not related to oxidative damage. They enhance differentiation, apoptosis, and growth inhibition of cancer cells, as well as inhibit or enhance gene expression and/or the activity of many proteins *. Antioxidants selectively inhibit the repair of radiation damage to cancer cells but protect normal cells, and there are no published studies showing that antioxidants protect cancer cells from radiation * *.
To assess the potential effect, it is worth making distinctions not only between doses and initial conditions, but also between classes of antioxidants, and even specifically between each of them. Endogenous antioxidants such as glutathione may increase the resistance of some cancer cells *; increase the metastatic potential of cancer cells *; as well as stimulate the transition of the cell cycle to the phase of DNA replication *. Exogenous antioxidants such as vitamins A, B, C, E were not seen in such effects.
In an attempt to reconcile the two opposing concepts regarding antioxidants, a science-based micronutrient complex has been proposed that can be used in conjunction with radiation therapy * *. It includes high (but not low) dietary antioxidants: buffered vitamin C, natural vitamin E, natural vitamin A, and natural vitamin B *, as well as some other bioactive supplements, with the exception of copper, iron, and manganese. The full composition of the patented * mixture «Optimal Health»:
- vitamin A (retinol palmitate) – 5'000 IU/day (2.75 mg/day);
- beta-carotene from seaweed – 75 mg/day;
- natural vitamin E (α-tocopheryl succinate) – 900 IU/day (800 mg/day);
- buffered vitamin C (calcium ascorbate) – 9'000 mg/day;
- vitamin D3 (cholecalciferol) – 400 IU/day (10 μg/day);
- thiamine mononitrate – 4 mg/day;
- riboflavin – 5 mg/day;
- niacinamide ascorbate – 30 mg/day;
- D-calcium pantothenate – 10 mg/day;
- pyridoxine hydrochloride – 5 mg/day;
- cyanocobalamin – 10 μg/day;
- folic acid – 800 μg/day;
- D-biotin – 200 μg/day;
- selenium (L-seleno-methionine) – 100 μg/day;
- chromium picolinate – 50 μg/day;
- zinc glycinate – 15 mg/day;
- calcium citrate – 250 mg/day;
- magnesium citrate – 125 mg/day.
The extensive list of micronutrients is explained by the fact that many of them can be depleted during radiation therapy.
Several other similar formulas have also been proposed.
Half the dose is taken in the morning and half in the evening because the half-life of most of these micronutrients is about 6-12 hours. This complex of nutritional antioxidants is proposed to be taken before and after irradiation – during the entire therapeutic period and, at least, a month after it.
In a 2004 pilot study (Phase I/II), this addition to radiotherapy significantly reduced the relative risk of tumor recurrence and metastasis in patients with stage 0-III breast cancer over a subsequent 22-month follow-up *. Of the 25 people in the control group, 2 relapsed, while the experimental group of 22 did not. Unfortunately, a small sample does not give a sufficiently convincing result, and it can be called into question; especially since no one has since proven such success in larger studies.
However, preclinical studies provide optimistic results. Vitamin C, α-tocopheryl succinate, and natural β-carotene at high doses markedly alter gene expression, protein levels, and the translocation of certain proteins. Which, in turn, induces differentiation, growth suppression and apoptosis of cancer cells, without having a similar effect on most normal cells * * *.
A mixture of dietary antioxidants – vitamin C (100 μg/mL), α-tocopherol (10 μg/mL) and β-carotene (10 μg/mL) itself slightly increased apoptosis, but greatly increased the apoptotic effect of paclitaxel and carboplatin in vitro *. The most pronounced effect was observed when the cells were treated with a mixture of antioxidants just before the start of chemotherapy, followed by paclitaxel exposure for 24 hours, and then exposure to carboplatin for 24 hours.
Summing up, we can say that an adequate supply of antioxidants is very important for preventing disease and maintaining health, but their excess does not provide noticeable benefits, and under many conditions can even be harmful. However, the same can be said about any other additives. Exceeding the physiological norm of endogenous substances, as well as the constant intake of exogenous substances in volumes significantly exceeding the volume that could come from food sources, is unjustified.
At the same time, during the period of anti-cancer therapy, ultra-high doses of exogenous antioxidants can enhance its effectiveness. However, in both the first and second cases, the result will depend on the initial level of free radicals in the tumor tissue.
Digestive enzymes have a multifaceted effect on the processes occurring in the body.
First of all, together with intestinal hydrochloric acid, they are involved in the breakdown of proteins consumed with food into amino acids. Amino acids are the basic building material for the synthesis of new proteins; unlike dietary proteins, they do not elicit an immune response. However, if the process of decomposition of proteins into components is incomplete – either because of the abundance of protein foods, or because of the lack of enzymes, then incompletely split foreign protein molecules enter the bloodstream through the small intestine, which are considered by the immune system as an invasion of pathogens.
The immune response to them increases the inflammatory index and contributes to the development of a number of diseases. The problem is aggravated with age, when the level of production of hydrochloric acid by the stomach and proteolytic enzymes by the pancreas falls; as well as in a condition popularly referred to as «leaky gut syndrome». Taking hydrochloric acid and digestive enzyme supplements may be a good idea for older adults and for people who have insufficient secretions of both. However, this decision must be agreed with your doctor.
Proteolytic enzymes, both produced in the body and taken orally, enter the duodenum. If this happens during a meal, then they are first involved in the digestion of food proteins. And only their excess (if any) is absorbed into the blood, in order to then re-enter the pancreas, completing its circuit. If enzymes are taken on an empty stomach, then they are absorbed into the blood in a significant amount, since they have nothing to spend on.
Once in the general circulation, the activity of proteolytic enzymes is limited by specific factors in the blood and other body fluids so that they do not damage the body's own proteins. Circulating throughout the body, the enzymes break down the protective protein layer of migrating tumor cells, exposing them for recognition and attack by immune cells. Thanks to this, the threat of metastasis, the main cause of death in cancer, is falling.
By dissolving thrombogenic fibrin, proteolytic enzymes also improve blood flow. They can also reduce levels of inflammatory molecules such as tumor necrosis factor (TNF), C-reactive protein (CRP), and circulating immune complex (CIC).
The activity of proteolytic enzymes depends on the acid-base state of the solution. And if we want to use them to fight against a malignant tumor, then the combination of enzymes with oxygen saturation and alkalization of the tumor will be more successful than using them alone.
Trypsin and chemotrypsin are the strongest antitumor proteolytic enzymes, but weaker enzymes such as bromelain, nattokinase, papain, and serrapeptase are also used.
• Wobenzym N, a complex containing the enzymes pancreatin, bromelain, papain, trypsin, chymotrypsin and rutin. Dosage: 2-3 tablets twice a day. Analogues: Megazime Forte (a combination of trypsin, chymotrypsin, bromelain and zinc), Wobe-Mugos (a combination of trypsin, chymotrypsin and papain), Swanson Ultra n-Zimes.
Biguanides reduce blood glucose and insulin levels and increase insulin sensitivity of cells, suppress protein synthesis in cancer cells by reducing aerobic energy production in mitochondria, reduce inflammatory potential, and significantly improve the gut microbial profile.
• Metformin. Just like aspirin, metformin is of plant origin. Metformin and other biguanides selectively inhibit cancer stem cells, prevent the transformation of stem cells into cancer cells *, reduce the overexpression of the HER2 oncoprotein *. Biguanides significantly enhance the effect of chemotherapy drugs * (27.5% vs. 6% complete regression *), as well as the effect of monoclonal antibodies against cancer stem cells *.
Long-term administration of moderate doses of metformin, beginning in middle age, increases insulin sensitivity of cells, lowers cholesterol levels and generally improves physical performance, reduces symptoms of aging and increases lifespan in mice *.
While tamoxifen in menopausal patients can lead to a 7-fold decrease in insulin sensitivity and an increased risk of type II diabetes mellitus *, metformin helps to reduce their risk of metabolic syndrome.
Metformin inhibits the proliferation of malignant cells under conditions of hypoxia, characteristic of tumors, without affecting the viability of non-malignant cells *.
Metformin also inhibits cancer cell proliferation in the presence of glucose, but induces cell death when glucose is removed *. From the latter observation, it can be concluded that metformin increases the dependence of cancer cells on glucose, and that the simultaneous use of metformin with restriction of glucose supply may be a successful treatment option.
The dosage for symptoms of diabetes averages 1'000 mg/day. The dosage in the treatment of cancer in the post-therapeutic period is 500 mg/day, and in the therapeutic period – 1'500 mg/day.
Bisphosphonates are used in the destruction of bone tissue, including due to metastasis and/or due to the use of aromatase inhibitors.
Breast cancer of the bone most often metastasizes to the bones (more than 30% of cases). Short-term use of bisphosphonates reduces the risk of bone fractures by 30-50%. Bisphosphonates not only inhibit bone metastasis. They can also markedly reduce relapse rates and improve overall patient survival, especially in postmenopausal women. Therefore, bisphosphonates can be used as an adjunct to clinical practice *.
Several studies have reported a 15% reduction in the risk of breast cancer as well as a 30% reduction in the risk of invasive cancer in bisphosphonate users *. However, bisphosphonates reduce blood levels of vitamin D, and to reduce this side effect, the combination of bisphosphonates with vitamin D is recommended * *.
• Zoledronic acid (zoledronate) is the best choice of bisphosphonates available today, but requires a 4 mg infusion every 6 months and has a number of negative side effects.
• Risedronate (35 mg once a week) taken for a year successfully controlled bone loss and bone density in women treated with chemotherapy *.
In addition to bisphosphonates, natural remedies can be used to protect bones from estrogen depletion. Thus, the combination of pomegranate and grape seed extracts induces the expression of genes associated with a decrease in bone resorption and counteracts bone destruction, and the combination of quercetin and licorice extracts induces the expression of genes that reduce osteoclast activity and promote bone growth. Both of these combinations can be alternated with short breaks, since the simultaneous use of all four plant components levels out the target effects *.
Hormones.
• Melatonin (a «sleep hormone») is extremely important for longevity, immunity and cancer prevention, especially hormone-dependent – breast and prostate. Melatonin inhibits both the initiation and development and metastasis of cancer, showing the widest spectrum of anticancer activity. Taken on an ongoing basis, it reduces the incidence and size of primary breast tumors in patients *. Taken during chemotherapy, it reduces the toxicity of chemotherapy drugs without reducing their therapeutic effect, and also weakens the primary and/or secondary (developed) drug resistance of the tumor *.
Melatonin is an endogenous substance, so it is not addictive, and has no toxicity and contraindications. Since it is a light-sensitive molecule, melatonin levels decrease due to the extension of daylight hours due to artificial light, which promotes the growth of cancers *. Conversely, shortening the light period plays against the risk of cancer. Breast cancer has been observed to be half as common in women with complete loss of light perception as in women with poor light perception *. The production of melatonin, like most other hormones, declines with age, making it reasonable to take exogenous melatonin at an older age, regardless of conditions.
Dosage: usually it is 0.3-3 mg in the evening just before bedtime. In any case, the dosage should not exceed 20 mg/day. Usually start with the smallest doses, and gradually increase them until the desired effect is achieved. It is recommended to dissolve the melatonin tablet under the tongue instead of swallowing. The time of taking melatonin should coincide with the time of the natural rise in its level in accordance with the daily rhythm.The time of taking melatonin should coincide with the time of the natural rise in its level in accordance with the daily rhythm. Taking melatonin in the morning instead of the evening can have the opposite effect, stimulating tumor growth *.
In addition to the introduction of exogenous melatonin, measures should be taken to prevent the destruction of endogenous melatonin caused by artificial lengthening of the light period. Since the blue part of the spectrum * signals the light period of the day to the photoreceptors of the retina, it can be removed from artificial lighting in various ways. To do this, in the evening, you can use warm glow lamps, amber colors for decorating the room, color correction of visual screens and, finally, glasses that filter blue light. There are even applications that remove the blue component from the screens of electronic devices in the evening.
The combination of these techniques may improve the quality of sleep by reducing the breakdown of melatonin *. Conversely, the blue shade of artificial lighting can be activated in the morning. At the same time, you need to sleep in complete darkness, without emergency lighting and nightlights, no matter how weak they may be. Even weak street light shining into the bedroom at night can increase the risk of breast cancer by lowering melatonin levels *. Cortisol is an antagonist of melatonin, so before going to bed, you should try to reduce its level.
S-Acetyl-Glutathione, Alpha Lipoic Acid, Milk Thistle, Selenium, and N-Acetyl Cysteine supplements may be helpful for people with BRCA mutations or a family history of cancer.
The above detailed review of supplements can cause some confusion – which ones, and in what quantity, should be used after all? The short answer would be:
• Taking proven supplements that the body is deficient in, unlike prescription drugs, is likely to be safe. While prescription medication is one of the leading causes of death worldwide *, only a few deaths have been reported due to incorrect supplementation.
• No supplements are a cure for cancer. They may be fundamentally necessary, but they cannot be a sufficient condition for the normalization of health. As their name implies, supplements can only supplement basic treatment or prevention.
• We eat to replenish energy, and drink water when its reserves in the body run low. But we do not eat or drink many times more than required. Similarly, the need to take vitamins, trace elements and minerals, as well as the dosage of a particular supplement, may be caused solely by their insufficient intake and/or low levels in the body, which can only be determined through appropriate laboratory tests. If there are no deficiencies, no supplements are required.
• The intake of supplements is stopped immediately after the normalization of the indicators that caused the need to take them.
• There are only a few supplements that can be justified regardless of current health conditions. They are caused either by our lifestyle, age, or genetic abnormalities, and are considered separately for each age group †.
• All scientifically recommended supplement dosages are based on population averages and do not take into account the individuality of each organism and the initial level of a particular vitamin and/or element. In addition, they are probably too low, and can only prevent clear signs of a shortage, but not always provide a real need for them.
• In any case, exogenous supplements, with a few exceptions, are taken for up to 3 months (up to a maximum of six months), after which a break should be taken †. Longer consumption of exogenous substances causes a loss of their effectiveness.
• The intestinal microflora synthesizes a large amount of vitamins, which can significantly adjust the dosage of vitamin K and B vitamins (thiamine, riboflavin, nicotinic acid, pantothenic acid, pyridoxine, biotin, folate, cobalamin *).
• Simultaneous intake of several additives can theoretically in some cases cause unpredictable consequences caused by the problem of their compatibility. In addition, a combination of additives acting in opposite directions will certainly reduce their effectiveness.