Health Strategy.
The human body does not exist on its own, but in homeostatic symbiosis with the microorganisms in it, the number of which greatly exceeds the number of all cells of the body itself, and the weight of which is about 3% of the body weight. Indeed, we are colonized by a huge number of microorganisms whose metabolism is mutually related to ours. Thus, our health is highly dependent on the mutual relationship of host cells (the body itself) and guests (foreign cells naturally present in it). The ecosystem of microorganisms, consisting of bacteria, viruses, fungi and protozoa, living in various parts of the body, is called the microflora (microbiota).
Harmonious cohabitation of the host and guests is mutually beneficial for both parties to the transaction. The body provides microflora with nutrients and a favorable environment. In response, the microflora helps form the protective lining of the gut and contributes significantly to human health.
The microflora did not just coexist with man throughout his history. She also evolved with him. As a result, strains of bacteria have proliferated that have a better interest in the health of the host than others, since their survival depends on it. For example, the bulk of the so-called «beneficial» bacteria produce short-chain fatty acids that feed intestinal cells, and have anti-inflammatory and antitumor properties.
The vast majority of micro-organisms-guests are concentrated in the large intestine. Therefore, the state of its microflora has a strong influence not only on the functions of the intestines and the immune system, but also on many other distant tissues and organs.
The gut is where much of the activity of the immune system takes place, and it is here that more than two-thirds of all immunologically active cells in the body are present. Gut-associated lymph nodes make a significant contribution to repelling invading pathogens. In addition to them, foreign cells lining the intestines take a significant part in the immune defense. They produce a range of molecules that can neutralize pathogens *. The microflora of the gastrointestinal tract is the main regulator of the immune system, not only in the intestines, but also in other organs *.
Colon bacteria ferment indigestible carbohydrates to produce short chain fatty acids (SCFA) such as acetate, propionate, butyrate * *. And SCFAs, through their cellular receptors, are able to suppress numerous signs of cancer, such as apoptosis, proliferation, cell invasion, and oncogene expression.
In addition, intestinal bacteria produce so-called postbiotics – vitamins, organic acids, lipids, as well as complexes of proteins and amino acids. Thanks to this, the body can receive a significant part of the micronutrients that were not received from food. Other metabolites (postbiotics) of intestinal bacteria can act as endotoxins or carcinogens and, by spreading throughout the body, deeply interfere with a variety of metabolic and signaling processes *.
Useful part of intestinal bacteria * binds bile acids *, produces essential amino acids *, B vitamins (B2, B7, B9, B12) and K *, promotes the absorption of minerals *, deactivates toxins * and carcinogens *. The harmful part is able to restore conjugated and ready to be removed estrogens, allowing them to re-enter the bloodstream and increase the risk of hormone-sensitive cancer subtypes *.
Maintaining a diverse and thriving population of beneficial gut bacteria helps curb the growth of harmful bacteria by competing for nutrients and colonization sites. Several compelling associations have been established between common chronic disorders and impaired bacterial composition and gut function *.
There is increasing confidence that intestinal symbionts play an important, if not decisive, role in human physiology and in the development of the most common chronic diseases, including cardiovascular diseases, obesity, chronic infections, depression, asthma, autism, and cancer. They may also act as an endocrine organ capable of regulating inflammatory, metabolic and infectious diseases * *.
In addition, bacterial diversity plays a critical role in both innate and adaptive immune function *. Finally, certain gut bacteria may increase the effectiveness of some traditional chemotherapy * and immunotherapy * anticancer drugs.
An unhealthy state of the intestinal microflora is a widespread problem. The diet of modern man is far from both the diet of hunter-gatherers and the diet of an agrarian society, which certainly affects the increase in the incidence of «diseases of the century» *.
While a healthy gut microflora is not the answer to all health problems, it can go a long way in helping to resolve them.
The predominant sections of bacteria in the healthy microflora of the duodenum are Firmicutes and Actinobacteria in the absence of Bacteroidetes, and in the jejunum Lactobacillus, Escherichia coli and Enterococci * predominate, which depends on the functional purpose of each of their sections of the intestine.
The two main divisions of bacteria found in the large intestine are the gram-positive Firmicutes (Clostridium, Ruminococcus, Enterococcus, Lactobacillus) and the gram-negative Bacteroidetes (Bacteroides, Prevotella). They account for up to 90% of all intestinal bacterial cells. The Firmicutes division produces butyrate, while the Bacteroidetes division produces acetate and propionate. Butyrate has been associated with a reduced risk of breast cancer, but equally important for health is the ratio between these short chain fatty acids.
The balance between these two groups of bacteria determines the healthy state of the intestines, and the whole organism as a whole *. An increase in the proportion of Firmicutes is directly related to inflammation, obesity, diabetes, and metabolic syndrome; and an increase in the proportion of Bacteroidetes is associated with an improvement in overall health and slim figure *. However, the optimal value of the Firmicutes:Bacteroidetes ratio is still a matter of controversy *.
The rest of the microflora of the large intestine is formed mainly by Actinobacteria (Propionibacterium, Micromonosporaceae) and Proteobacteria (Escherichia, Helicobacter, Rickettsia) divisions. The proportion of each specific type of intestinal bacteria (bacterial profile), despite the general similarity for each organ, varies slightly from person to person. These individual differences are usually stable and persist throughout a person's life. However, the gut microflora may differ slightly between men and women due to the influence of sex hormones *.
This whole complex ecosystem coexists in a delicate balance that can be easily disturbed, thereby creating dysbiosis. The main destroyer of the well-being of the biocommunity is usually called antibiotics. Indeed, women who received up to 25 antibiotic prescriptions over a 17-year follow-up had a 50% higher incidence of breast cancer than women who did not take antibiotics. And for women who have written more than 25 prescriptions – twice as much *. However, many other disruptors actually exist, including some drugs (such as tamoxifen), toxins, infections, and inadequate food.
Even a simple change in food composition can cause a rapid change in the bacterial profile. If plant foods don't degrade microbial diversity, then animal foods can do it fairly quickly *. For example, switching from a vegetarian to a carnivorous diet results in a change in the profile of gut bacteria in as little as 24 hours *.
In parallel with such phenomena as the systematic predominance of junk food, the onset of menopause, an increase in body mass index, aging and the development of cancer, the bacterial profile of the intestine undergoes pathological changes associated with the impoverishment of the species diversity of bacteria and a change in their quantitative ratio * * *. For example, the Firmicutes:Bacteroidetes ratio is approximately 3:1 in lean individuals, while it can be as high as 30:1 in overweight individuals.
Dysbiosis is also associated with the risk of various types of degenerative diseases, including breast cancer *. In particular, breast cancer is accompanied by a change in the balance within the Clostridiales family, such as an increase in the proportion of Clostridiaceae (Faecalibacterium) and Ruminococcaceae, and a decrease in the proportion of Lachnospiraceae (Dorea) *. The most dramatic deterioration in the species diversity of symbionts is observed already at the very beginning of the development of breast cancer (stages 0-I) *, and as the disease progresses, it continues *.
Reducing the quantity and quality of the intestinal microflora, caused, for example, by the use of antibiotics, increases the risk of breast cancer *. Conversely, cancer patients with greater gut diversity exhibited higher progression-free survival compared to patients with low or moderate microflora diversity *. Thus, maintaining the richness of the intestinal microflora in the most favorable way affects the general condition of the whole organism.
However, we cannot ensure that the microorganisms we desire live in the intestines if we cannot provide them with comfortable living conditions, first of all, food. We can support gut bacterial health through dietary intake through adequate intake of plant fiber and plant polyphenols.
It is not surprising that for the Firmicutes:Bacteroidetes ratio, the source of protein and fat consumed is important *. The evolutionary plant-based diet of primates improves this ratio, while the animal-based diet worsens it * *. In addition, within the Bacteroidetes division itself, plant foods beneficially increase the ratio between the genera Prevotella and Bacteroides *.
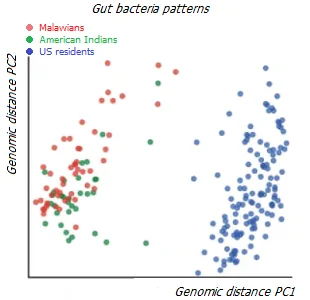
It is interesting to compare data from the intestinal microflora of populations around the world leading different lifestyles – hunter-gatherers, farmers, pastoralists, herders and urban residents *. An analysis of microbiome diversity has shown that the bacterial composition of the gut reflects diverse lifestyles, representing a transition from hunter-gatherers to industrialized populations. As expected, the populations of the industrialized regions are similar in profile and have a lower diversity of gut microorganisms than the non-industrialized populations.
However, in different categories of the non-industrial population, the microflora demonstrates different species diversity. In particular, the bacterial profile of pastoralist and agropastoral populations is more similar to that of urban populations than to that of hunter-gatherers. At the same time, the profiles of modern hunter-gatherers living in different geographical and environmental conditions are more similar to each other than to the bacterial profile of US residents on a «Western» diet *.
In fact, eating a diet that supports the diversity and abundance of beneficial gut bacteria is critical to immune health *. The more food we provide for our gut symbionts, the better conditions for them to thrive. The more diverse the consumption of plants, the richer the intestinal microflora. Therefore, at least 3 kg of 30 different plants per week is recommended.
Symbionts require both soluble and insoluble fiber, so we should consume not only fruits, vegetables, roots, tubers and stems, but also whole grains and legumes, nuts and seeds. It will also improve the body's supply of plant polyphenols, vitamins and certain chemical elements. Switching to a low-calorie vegetarian diet can benefit the gut by reducing body fat mass *.
In addition to changing our diet, we can adjust microbial richness and diversity by taking bacterial supplements called probiotics.
Probiotics. Probiotics, by definition, are non-pathogenic microorganisms for humans, which, when administered in sufficient quantities, are able to form a healthy microflora of organs, as well as have a detrimental effect on pathogenic and opportunistic bacteria. For each person, depending on their specific condition, specific strains of probiotics may be required. However, there are some universal strains of bacteria that are equally beneficial in improving the overall health of most people.
Most attention has traditionally been given to Bifidobacterium and Lactobacillus, which are the dominant species in breastfed children but of limited value in adults. A number of recent clinical studies have revealed the unconditional benefits of Streptococcus thermophilus, Bifidobacterium longum, Bifidobacterium breve, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei, Lactobacillus bulgaricus. Currently, new promising candidates have been added to them, such as Ruminococcus bromii, Roseburia intestinalis, Eubacterium recttale, Faecalibactrium prausnitzii. In addition, it turned out that the effectiveness of probiotics in promoting health depends more on the strain than on the type of bacteria.
With an adequate diet, there is no absolute need for regular intake of probiotics; however, from time to time they have to be resorted to. For example, after cleansing enemas, with stool disorders, during and/or after taking antibiotics, with a sharp change in diet (for example, on a foreign tour), with a prolonged lack of fiber, or due to other events that negatively interfere with the life of the intestinal microflora. While the disruption of the bacterial balance in the gut can be very rapid, it may take at least 2 weeks for it to fully recover.
The task of probiotics is not to provide the necessary mass of intestinal bacteria; such a goal can only be achieved by providing enough food for these bacteria. The task of probiotics is to provide the necessary diversity of intestinal bacteria; Taking probiotics periodically will help to maintain an adequate bacterial profile in the gut. However, probiotics should not be abused unless absolutely necessary. They are used as a temporary measure for dysbiosis in the same way that plaster fixation is used as a temporary measure for a broken bone.
Probiotics may have a specific purpose that is not limited to colon health. For example, a mixture of Lactobacillus helveticus Rosell-52 and Bifidobacterium longum Rosell-175 can significantly improve mood, stress response and emotional balance * *. And Lactobacillus rhamnosus CRL 1505 stimulates the immune system in the respiratory tract and intestines *. In general, the effect obtained depends on the bacterial composition of the probiotic mixture.
There are a large number of probiotic offerings on the market from many vendors. However, not all of them have been tested and proven effective in clinical studies. Even many popular fermented milk products may not meet the level of scientific evidence required. Few strains of bacteria can be called true probiotics, because the nature of the action of each strain is as different from the others as the characters of a dog breed differ from each other.
An important tip is to purchase only those probiotics that have clinical evidence of their effectiveness. It should be ensured that the strains of microorganisms present in the probiotic are suitable for achieving the goal, and their number corresponds to the recommended results of clinical studies.
As supplements, you should choose enteric capsules containing at least 8 different types of bacteria, while each of them should be at least 6 billion units. When choosing a probiotic, preference is given to dry lyophilized bacteria (even better – spores of bacteria) in enteric capsules, since after ingestion they have to go a long way to the intestines through the aggressive contents of the stomach and duodenum. Many of them are offered as part of ready-made bacterial complexes:
• Vivocaps forte™ contains all of the above clinically tested bacterial strains with proven efficacy. Dosage: 2 caps/day for at least 2-4 weeks. The courses are repeated 1-2 times a year to maintain the vital activity of the microflora, and each time to restore it after taking antibiotics, food poisoning, deep cleansing enemas, digestive disorders, expulsion of helminths and other events and procedures that negatively affect the intestinal microflora. Probiotics are usually combined with vitamins В9 and В12.
• Prescript-Assist™ contains approximately 30 soil micro-organisms typical of a healthy gut. Recommended for enrichment of bacterial diversity. The complex has shown clinical significance in irritable bowel syndrome * *. Dosage: 2 caps/day for 2 weeks to 2 months.
• Lactovit Forte™ contains spores of antibiotic-resistant lactobacilli Lactobacillus sporogenes in combination with vitamins В9 and В12, which are required for their development. This complex is recommended during and after gross interference with the intestinal microflora (antibiotics, pathogenic bacteria, enemas). One limited clinical study showed an improvement in symptoms in patients with dysbiosis after a month of taking Lactovit Forte *. Dosage: 2-4 caps/day for 4 to 6 weeks.
• BioGaia ProTectis™ contains lactobacillus Lactobacillus reuteri, which is involved in the formation of the microflora not only of the entire gastrointestinal tract, but also of the urinary tract, skin and mammary gland. It strengthens the intestinal cell barrier, suppresses the action of pathogenic microorganisms and reduces the level of local inflammation. Although there are a number of publications in favor of Lactobacillus reuteri in various disorders of the gastrointestinal tract, the results of clinical studies of the BioGaia supplement itself have not been reported. Dosage: 1-3 tab/day.
• Boulardi™ contains the antibiotic-resistant yeast fungus Saccharomyces boulardii, which suppresses pathogenic microorganisms in gastrointestinal disorders, and also improves immunity by stimulating the production of immunoglobulin. Clinical studies have found the fungus to be safe and effective for the prevention and treatment of gastrointestinal disorders *. Dosage: 1 pack/day.
• EM-curunga is a complex of bifido- (Bifidobacterium spp.) and lactobacilli (Lactobacterium spp.), sour-milk streptococci (Streptococcus lactis, Streptococcus cremoris) and yeast (Torolopsis). It has antagonistic activity against Helicobacter, Staphylococcus and Escherichia coli. 1 g of dry concentrate or sourdough contains 104 bacteria.
Many foods also support gut bacterial diversity, live yogurt or kefir, sauerkraut, kombucha, natto, and other live fermented foods.
However, there is a fundamental difference in whether bacteria colonize a sterile environment, such as the intestines of a newborn, or whether they colonize an already existing community of competing bacteria that have already built relationships. In the latter case, a strong and persistent intervention may be required to change the bacterial profile, because the established microflora may not accept «newcomers».
Again, sowing life into barren soil will be a futile exercise. To create a healthy intestinal microbial community, it is necessary, in addition to the probiotics themselves, to provide them with nutrients (prebiotics). The bacterial profile of the gut depends more on the bacterial food that is introduced into it than on the ready-made bacteria that are introduced into it. The total weight of gut bacteria is measured in kilograms, while the weight of supplements taken is measured in grams. Thus, supplements can provide a variety of beneficial bacterial species, but supplements are unlikely to significantly help bacteria grow if there is no food source for their growth.
Thus, reducing caloric intake * or eating prebiotic-rich foods (as fiber) is a regular measure to ensure gut health, while taking probiotics is an emergency and corrective measure.
Prebiotics, by definition, are non-absorbable substances that nourish the healthy gut microflora. Prebiotics perform mechanical cleansing of the intestines, provide a favorable environment for the reproduction of intestinal bacteria, bind carcinogens, speed up the passage of food through the intestines, and reduce the risk of breast cancer *. Unlike other components of the intestinal contents, prebiotics are able not only to form a bacterial spectrum, but also selectively support the beneficial part of the microflora.
Various groups of bacteria in the intestinal environment are constantly fighting for survival and ousting competitors. A similar picture is observed in other organs with a mucous membrane. By generously feeding bifidobacteria and lactobacilli, we provide our beneficial symbionts with more comfortable conditions for prosperity and dominance. And accordingly, less favorable conditions for pathogenic microorganisms.
Each type of bacteria prefers some kind of food source. Therefore, the number and bacterial spectrum of the intestinal microflora directly depend on the composition of the food taken * *. Bacteria associated with a high risk of cancer prefer animal protein and fat, and produce hydrogen sulfide. And low-risk bacteria prefer fiber and produce butyrate.
Prebiotics are mainly fiber and resistant (to the action of digestive enzymes) starch. Fiber can be water-soluble (such as oats, legumes, nuts, berries) and water-insoluble (such as cereal shells).
Soluble fiber includes pectin found in apples, carrots, gooseberries, oranges, pears, plums, quince; inulin contained in cereals, onions, bananas, garlic, asparagus, in the roots of Jerusalem artichoke and chicory; beta-glucan contained in the grain shell; gum and mucus contained in the resins of trees or, for example, in oatmeal.
Insoluble fiber includes lignin, cellulose, and fructooligosaccharides.
The recommended intake of soluble fiber for women is from 25 g/day, and insoluble – from 50 g/day. Reconstruction of the Paleolithic diet shows that the ratio of insoluble:soluble fiber was approximately 1:1 with a total intake of 150 g/day *.
An excessively high intake of coarse fiber can have negative consequences, such as irritating and even traumatizing the intestines, increasing the risk of colon cancer. In addition, excess fiber can reduce the intake of valuable minerals such as calcium, phosphorus, magnesium and potassium, both due to their binding and removal by fiber, and due to too rapid removal of food mass from the intestines.
The source of fiber is plant foods – whole grains and cereal bran (wheat, rye, barley, oat, etc.); dried fruits; legumes; as well as ground flaxseed, which inhibits tumor growth by stimulating cell differentiation and reducing the expression of growth factors ILGF and EGF. It has been clinically found that the most beneficial fiber is derived from grains *.
Bran in the form of a dietary supplement can solve several problems at once: providing inositol, fiber, trace elements and beta-glucan. A potential problem is that bran, like grain in general, can be contaminated with mold toxins, pesticides, or other carcinogenic contaminants. Therefore, choosing a reliable supplier is very important.
Another problem is that cereal bran contains lectins such as gluten, which can cause inflammatory or autoimmune reactions in some people who are sensitive to it. In this case, low lectin bran, such as psyllium bran, may be an alternative. A less preferred fiber supplement is powdered inulin.
Most prebiotics that have the ability to stimulate bifidobacteria are neutral carbohydrates. The most studied of them are fructooligosaccharides (FOS), transglycosylated oligosaccharides (TOS), soy oligosaccharides (SOS), lactulose. Examples of sources of FOS are Jerusalem artichoke, chicory, bananas, figs, onions.
Intestinal permeability inhibitors. The integrity of the intestinal mucosa is critical to health. The weakening of the intestinal barrier (the so-called «leaky gut») contributes to the entry of not only potential toxins from the intestine into the blood, but also potentially dangerous microorganisms.
This increases both local and global inflammatory levels, contributing to the development or exacerbation of many degenerative diseases including Parkinson's disease, autism, cardiovascular disease, metabolic syndrome, kidney and liver disease, type II diabetes, obesity, and others. In addition, intestinal bacteria, penetrating into the general circulation and spreading further throughout the body, can significantly change the bacterial profile of other organs.
Intestinal permeability can increase under the influence of many factors, including aging; radiation and chemotherapy; infections and inflammation; low levels of thyroid hormones; taking antibiotics, acetylsalicylic acid, vitamin C and proton pump inhibitors; lack of fiber, zinc and vitamins A and D; alcohol, a pro-inflammatory diet, and food allergens.
Deglycyrrhizinated licorice extract, slippery elm bark, and marshmallow root can be used to strengthen the intestinal mucosa. Vitamin D, fish oil, polyphenols from grape seed extract, L-glutamine (a protein that feeds intestinal cells) also help restore the strength of the intestinal walls.
Metformin. Long-term use of metformin causes significant positive changes in the bacterial profile of the intestinal microflora *, which are accompanied by an increase in the production of butyrate.
Meanwhile, the healthy balance of intestinal microflora is influenced not only by the composition of the food consumed. There are many other factors that have an indirect effect; for example, stomach, liver, pancreas, and thyroid health. Hypothyroidism, as well as a lack of hydrochloric acid or bile salts, can cause intestinal dysbiosis. And without addressing these health issues, addressing dysbiosis may be ineffective. In the body in general, a lot of things are interconnected by various mechanisms, which are not always clearly expressed.
For a long time, many tissues of the body were considered sterile, and the presence of any foreign microorganisms in them was considered as an infection. Recently, this view has undergone significant revision. Virtually all of the body's mucous membranes are colonized by bacteria that play the same role in their morbidity, defense, and function as bacteria in the gut. And each organ, including the mammary gland, has its own specific bacterial profile.
Bacterial profiles in breast tissues differ between healthy women and women with breast cancer. Each subtype of breast cancer has been found to have unique bacterial, viral, fungal and parasitic profiles *. At the same time, tissues of ER+ and HER2 tumors have similar microbial signatures, while TNBC tumors show a wide variety of microbial signatures *.
Identification of a breast-specific microbiome has found that healthy breast tissue, as well as tissue from all four cancer subtypes, is dominated by bacteria such as Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes. However, healthy tissues contain more Lactococcus and Streptococcus *. And malignant tissues are relatively enriched in many other potentially pathogenic microorganisms * – Bacillus, Staphylococcus and Enterobacteriacae *. It is known that populations of Enterobacteriacae and Staphylococcus are able to cause double DNA strand breakage, leading to genomic instability, similar to Helicobacter pylori, the recognized culprit of gastric cancer.
Different types of bacteria can convert the same feedstock into different end products. Therefore, the state of her health depends on the outcome of the interspecific competition of bacteria located on the epithelial layer of the ducts and lobules of the mammary gland. Pathogenic microorganisms are able to provoke inflammation and exhibit carcinogenic effects, while some beneficial strains of bacteria (such as lactobacilli) have, on the contrary, anti-tumor effects *. In addition, lactobacilli are able to suppress the growth of pathogenic fungal organisms.
Changes in the bacterial profile of the breast and the development of cancer in it go in parallel. And although there may be a mutual influence between them, cancer seems to be a consequence rather than a cause of it. Despite the external insignificance of these changes, they are accompanied by catastrophic consequences.
We do not have an effective ability to selectively reduce the level of unwanted strains of microorganisms in the breast epithelium in a direct way. However, we have the ability to regulate the bacterial profile of tissues indirectly, by maintaining the desired varieties of microorganisms in the colon.
Bacteria living in the intestines are known to be able to penetrate the intestinal wall into the bloodstream and reach distant sites, including the breast * *, and even the brain. That is, the bacterial composition of the intestinal microflora is able to influence the bacterial composition of the microflora of other organs, and thus their health and functionality. Apparently, for this reason, dysbiosis in women with breast cancer is systemic. Their bacterial imbalance is not limited to the intestines, but simultaneously affects the breast, urinary tract * and, apparently, many other organs and tissues.
The main factor determining the diversity of microorganisms in the intestines and, accordingly, in the mammary gland, is diet. In a manipulation study, it was found that monkeys on the so-called «Mediterranean» diet, the number of Lactobacillus in the mammary gland increases in comparison with monkeys by the so-called «Western» diet. What's more, the mammary glands of monkeys fed a «Mediterranean» diet showed higher levels of bile acid metabolites and increased amounts of postbiotics, biologically active compounds produced by gut bacteria * *.
A number of preclinical animal studies show * that regular intake of appropriately formulated probiotics can influence the bacterial spectrum of the mammary gland epithelium, inclining it towards an anti-inflammatory, antitumor side. Oral ingestion of acidophilus (Lactobacillus acidophilus) exhibits antitumor activity in mice with pre-existing breast tumors *.
One observational study found that as little as 0.5 cups a day of fermented dairy products could reduce a woman's chance of developing breast cancer by one-third, compared with controls, regardless of age and all other risk factors. And 1.5 cups (225 g) cuts the risk in half *. It is amazing how such a simple and cheap intervention can produce such beneficial effects.
Unfortunately, there are very few clinical studies on the relationship between breast cancer and probiotic intake. However, it can be assumed that supplementation with microorganisms such as Lactobacillus acidophilus, Lactobacillus Casei, Lactobacillus crispatus, Lactobacillus rhamnosus, Lactobacillus plantarum, Lactoccocus lactis, Enterecoccus lactis, Enterococcus faecalis, Staphylococcus hominis, Streptoccocus thermophilus andLactobacillus delbrueckii, can correct in a positive way. side bacterial profile not only of the mammary gland, but also of other organs *.
Many of these microorganisms are found in probiotic formulas:
• Vivocaps forte™. Multi-strain concentrated probiotic containing 4×1010 lyophilized lacto- and bifidobacteria.
• Back-Set Forte™. Multi-strain probiotic. At a dosage of 9×1010 bacteria, it protects the mammary gland from inflammation * thanks to the immunostimulating bacteria Lactobacillus fermentum and the anti-inflammatory bacteria Lactobacillus salivarius *.
• Probiotic-30™. «New Food» company multi-strain probiotic containing Streptococcus thermofilus and Lactobacillus salivarius. Probiotic-30™.
• Primal Defense Ultra®. Probiotic providing 5×109 bacteria per capsule from 13 different species including Saccharomyces boulardii, Lactobacillus plantarum, Bacillus subtilis, Bifidobacterium lactis, Bifidobacterium bifidum, Lactobacillus rhamnosus, Bifidobacterium breve, Lactobacillus casei, Lactobacillus salivarius, Lactobacillus acidophilus, Lactobacillus brevis, Bifidobacterium longum, and Lactobacillus paracasei. The probiotic has been clinically tested in women with breast cancer * (3 capsules at bedtime for 4 weeks), but the results are not yet known.
It is easy to see that the same groups of bacteria have a positive effect on both the intestines and the mammary gland. Thus, by taking care of our intestinal pets, we are doing a great service to our mammary gland at the same time.
Fungi. Bacteria are not the only type of microorganisms that colonize our organs. Until now, most attention has been focused on bacteria, while very little attention has been paid to fungi. Meanwhile, fungi were found in 35 types of cancer and were often intracellular. Moreover, each type of tumor has a characteristic fungal profile, and the number and predominant type of fungi allow predicting the clinical outcome of the disease *.
Among all previously untreated tumors studied, the highest amounts of fungal DNA were observed in bone and breast cancers. With approximately equal fungal diversity, Sporobolomyces roseus, Cladosporium and various Aspergillus species predominated in the tissues of a healthy mammary gland, in the tissues of a benign tumor – Yarrowia porcina, Aspergillus glabripes and Aspergillus penicilliodides, and in the tissues of a malignant tumor – Malassezia arunalokkei, Malassezia restrikta and Malassezia globosa. Malassezia globosa has been found to correlate with shorter survival in breast cancer *.
It cannot, of course, be said that fungi are the cause of the tumor. However, there is no doubt that they occupy an important position in the tumor microenvironment and enter into complex relationships with tumor cells and tumor bacteria, and the development of the disease depends on these relationships. Although fungi are quantitatively inferior to bacteria, their role is no less significant than the role of bacteria. Because different types of fungi induce unique immune responses in the host, and some of them are able to deactivate immune cells * * *, their presence in a tumor can make immunotherapy ineffective.
Our knowledge of the activity of fungi still requires serious consideration of how we can use them to improve the health of breast tissue. What forces the breast microflora to deform species dominance; should we specifically suppress Malassezia or force infection with Sporobolomyces roseus to restore the normal fungal profile; how changing the fungal profile can affect the bacterial profile and vice versa; and which microflora profile is fundamentally the healthiest – we will have to look for answers to all these questions in the coming years.