Health Strategy.
All of the previous points provide a background favorable to breast health. They are necessary, but may not be sufficient to control the growth, invasion, and metastasis of a tumor that has already formed. Below we will consider manipulations aimed directly at the tumor.
The destruction of cancer cells is central to modern oncology. The current standard of care for breast cancer includes surgery, radiation, and chemotherapy drugs such as cisplatin, paclitaxel, carboplatin, bevacizumab, doxorubicin, cyclophosphamide, docetaxel, and epirubicin *.
Many non-protocol natural substances are able to enhance the effect of chemotherapy, or destroy cancer cells on their own. Some of them are used in various clinics of complementary or alternative medicine. And although they may show much milder side effects, they are unlikely to be more effective than clinically used agents *.
Vitamin C, administered as a high-dose intravenous infusion, is one of the remedies long ignored by orthodox medicine. Therapy with this natural, non-toxic and cheap substance has the potential to be useful in the treatment of stubbornly refractory tumors * without the side effects typical of conventional chemotherapy.
Vitamin C in low doses has an antioxidant effect, but in high doses it has a pro-oxidant effect *. However, the creation of a high concentration of vitamin C is associated with the problem of its administration. Oral intake of even the maximum tolerated dose (18 g) of vitamin C cannot create its plasma concentration higher than 220 µM, while it can be increased to 26 mM by intravenous administration * *. These high levels of vitamin C produce extracellular and intracellular amounts of hydrogen peroxide, a powerful oxidant that is selectively cytotoxic to cancer cells in vitro * * * *.
Cancer cells are more sensitive to oxidative stress because they are already overloaded with oxidants. In addition, vitamin C produces free radicals in another way – by reducing metal ions such as copper or iron (Fenton's reaction). And since iron concentration is increased in cancer cells, oxidative stress is further increased for them.
The selective defeat of cancer cells by vitamin C is explained by their increased consumption of glucose. Accordingly, the number and activity of glucose transporters in glycolytic cells is greatly increased. It is known that vitamin C is transported into the cell by the same transporters as glucose * * *, and therefore the consumption of vitamin C by glycolysis cells is several times more active than its consumption by normal cells.
Although ascorbate alone is significantly less effective in inhibiting tumor growth than chemotherapy, some clinical studies show a clear benefit of combining ascorbate with radiation * and chemotherapy *. In an acidic, metal-rich tumor environment, ascorbate acts similarly to ionizing radiation, killing cells by damaging DNA with free radicals. High-dose ascorbate depletes intracellular reserves of antioxidants, so it can also be effectively combined with other therapies based on increasing oxidative stress.
Taken by itself, high-dose ascorbate is not able to reverse the process of carcinogenesis. However, it can reduce the side effects of chemotherapy * and increase patient survival *. In addition, ascorbate reduced leukocyte decline, weight loss, hepatotoxicity, cardiomyopathy, and many other chemo-induced side effects in animals * * * *.
Antitumor properties of vitamin C are not limited to its cytotoxic effect. Some in vitro studies have shown that high-dose ascorbic acid, especially in combination with selenium, promotes the return of cells in a precancerous state to normal metabolism * * *, apparently by suppressing anaerobic respiration *; and also inhibits angiogenesis * and promotes cell differentiation *.
In order to avoid acidification of the blood caused by ascorbic acid, it is recommended to use its salts (ascorbates), such as sodium ascorbate, and even better, potassium ascorbate, for intravenous infusions.
There is no approved protocol for the clinical use of this therapy, but some alternative treatment clinics use the Riordan protocol * *.
Current recommendations include the following: ascorbate dosage should be around 1g/kg; the frequency of administration should be at least 2 infusions per week; the duration of therapy is approximately 3 months, after which it will be possible to evaluate its effectiveness *.
In one clinical study * at an infusion rate of 0.5 g/min, the initial dosage for the first 2 sessions was 15 g vitamin C with an appropriate amount of carrier fluid. For the 3rd and 4th sessions – 25 g of vitamin C and 200 mg of magnesium chloride to prevent vascular spasm. For the 5th and 6th sessions – 50 g of vitamin C and 200 mg of magnesium chloride. For all subsequent sessions, 75 g of vitamin C plus an appropriate amount of carrier fluid and 200 mg of magnesium chloride. For patients with extremely aggressive tumors, the dosage was increased to 100 g of vitamin C and 400 mg of magnesium chloride. The target plasma vitamin C concentration was approximately 350 to 450 mg/dL. The course of treatment was 21 days of daily intravenous infusions, followed by infusions every 2-3 days a week, until the final effect was achieved. In addition, the additions listed three paragraphs later were used.
In this study, three out of four patients with ER+ breast cancer who adhered to the treatment protocol had a stage II primary tumor halved from baseline measurements within 3 weeks of treatment, and two of these three patients subsequently had complete regression.
Ascorbate is very rapidly metabolized, and its level falls sharply already 2 hours after administration. Therefore, a method in which 60 grams of ascorbate (in 500 ml of water for injection) is injected into a vein over 60 minutes, and then another 60 grams is slowly and continuously injected over the next 6 hours, will be more effective. Such a solution will make it possible, without sharp jumps in the concentration of ascorbate, to achieve a longer saturation of plasma with it at a sufficiently effective cytotoxic level *. Continuous constant infusions allow to further reduce the effective therapeutic dosage of vitamin C – up to 20 g/day *.
The addition of lipoic acid to ascorbate in a ratio of 1:10 made it possible in vitro to reduce the lethal dose (LC50) for cancer cells by a factor of 6, at least for rectal cancer cells. Other high-dose ascorbate performance enhancers are vitamin K3 *, coenzyme Q10, acetyl-L-carnitine, magnesium, aspartate, vitamin B complex, and low-dose copper supplements *. A high oxygen content in the blood is essential, from which vitamin C produces free radicals. Thus, an increased effect should be expected when this therapy is combined with hyperbaric oxygenation.
Other supplements that enhance the effectiveness of vitamin C therapy *:
- a diet low in sugar, carbohydrates and meat and high in fresh vegetables and fruits;
- gastrointestinal health supplements: pancreatic enzymes (400-1'200 mg/day), probiotics (9×109 units/day);
- antioxidant supplements: α-lipoic acid (600-1'200 mg/day), niacin (500-1'500 mg/day), resveratrol (10-20 mg/kg/day);
- fortifying supplements: CoQ10 (150-300 mg/day), cobalamin (80-100 mg/day), folic acid (0.5-1 mg/day), thiamine (5-30 mg/day);
- immune supplements: vitamin D (5'000-10'000 IU/day), vitamin E (0.05 mg/day), selenium (200 µg/day), zinc (50 mg/day), β-glucan (100-200 mg/day);
- anti-inflammatory supplements: curcumin (1-3 g/day), linseed oil (3-6 g/day), quercetin (0.5-1 g/day), boswellia (1.2-2.4 g/day), silymarin (300-900 mg/day).
A contraindication to ascorbate therapy is deficiency of the enzyme glucose-6-phosphate dehydrogenase (G6PD), which occurs in 0.5% of the population. Significant negative side effects of long-term high-dose vitamin C supplementation include: increased blood clotting; possible deficiency of vitamin B12, folic acid, copper and iron; inhibition of the pancreas; increased blood pressure; impaired renal function and possible growth of kidney stones.
The question of the possibility of acquiring drug resistance against ascorbate by cancer cells remains open. Another difficult issue is the general excessive increase in the amount of free radicals produced by ascorbate in the body. Because its selectivity is based only on its higher uptake by cancer cells, high-dose ascorbate can be potentially toxic to normal cells in the body that are prone to high glucose uptake. Local delivery of ascorbate could alleviate this problem, but this mode of administration has not yet been reported.
Therapy with high-dose ascorbate infusions is only possible in a clinical setting under the guidance of qualified personnel, and it is used only in alternative or complementary treatment clinics. As monotherapy, ascorbate is not a cure for cancer, however, as part of a comprehensive treatment program, ascorbate improves the quality of life of cancer patients and increases their chances of survival.
The problem that hinders its widespread use is the lack of a reliable positive evidence base obtained in the course of extensive clinical trials. But this is a common problem for all simple, cheap and obviously non-patentable medicines to enter the market. There is no one among individuals and organizations willing to spend billions of dollars in research without the hope of making a corresponding profit, and governments prefer to spend funds for other purposes.
Amygdalin, contained in the kernels of stone fruits, is a well-known complementary medicine. He became widely known thanks to the scandalous story when the pharmaceutical business, frightened by the possible loss of profits, compromised medical science.
Like ascorbate, amygdalin uses their increased appetite for glucose to selectively target cancer cells. However, in addition to the oxidizing one, he also uses toxic weapons.
The amygdalin molecule contains two glucose molecules, due to which it is easily transported into the cell, where, as a result of metabolism, it splits into molecules of glucose, benzaldehyde and the CN-ion of hydrocyanic acid. The last two molecules are cellular toxins. Cyanide, by binding to the respiratory enzyme cytochrome oxidase, blocks mitochondrial respiration of the cell, while benzaldehyde blocks glycolytic respiration *.
In the presence of oxygen, benzaldehyde is oxidized to form benzoic acid, which is then eliminated through the kidneys. However, during hypoxia observed inside the tumor, benzaldehyde remains unoxidized and accumulates. If the cyanide molecule, due to its small size, is easy to leave the cell, then it is more difficult for benzaldehyde to do so. If, at the same time, the activity of membrane pumps pumping toxins out of the cell is also suppressed, then the toxic effect of benzaldehyde will apparently increase.
Due to the strong oxidative stress caused by the action of amygdalin *, as well as due to the increase in the ratio of pro-apoptotic proteins to anti-apoptotic proteins, breast cancer cells, regardless of their hormone sensitivity, die by apoptosis *.
Clinical studies show that amygdalin also has anti-inflammatory * and analgesic * effects. In addition, it stimulates the immune system *. The results of experiments in vitro and using laboratory mice showed the potential benefit of amygdalin against tumor metastasis *.
Phase II clinical trials conducted in Mexico included 1'200 patients with advanced malignancies who were treated with amygdalin at various dosages. Intravenous infusions of 6-9 g of amygdalin over 20 minutes demonstrated an antitumor effect. Complete remissions, partial relief and long-term stabilization were observed in almost 33% of patients, who in more than 70% of cases were no longer candidates for conventional treatment. Subjective improvements were observed in more than 45% of cases – an improvement in the general condition of the patient, an improvement in appetite and a decrease in pain. Virtually all patients were able to switch from morphine derivatives to non-narcotic painkillers less than four weeks after starting amygdalin *. However, this work has not been published in peer-reviewed journals.
Even if such results were overestimated, amygdalin therapy may help the patient to endure the burden of the disease and enhance the effect of other therapies. Amygdalin does not provide long-term effects, therefore, requires constant use. By itself, it is much weaker than standard chemotherapy drugs, does not provide strong effects in terms of curbing tumor growth, and is not capable of leading to its regression.
The combination of amygdalin with other drugs may enhance its antitumor effect. Such an amplifier can be the following combination:
- a complex of pancreatic enzymes, such as Wobenzym™ (3 tablets two hours after each meal);
- vitamins A (1 mg/day), C (100 mg/day), D (50 mg/day), E (15 mg/day);
- zinc, selenium and iodine;
- omega-3 fatty acids;
- methylsulfonylmethane;
- vegetable antioxidants and herbs;
- freshly squeezed vegetable juices.
As in the case of vitamin C, a serious disadvantage of this therapy is the need to administer amygdalin by intravenous infusion, because taking amygdalin orally leads to the breakdown of its molecule already in the acidic environment of the stomach, and then in the intestines. As a result, cyanide enters the general bloodstream and poisons the entire body, instead of getting into cancer cells and poisoning only them. This explains the paradox that while injecting 6-9 grams of amygdalin safely provides a therapeutic effect, then oral administration of already 3 grams of amygdalin can cause severe cyanide poisoning *.
Possible side effects of amygdalin therapy include a slight drop in blood pressure, an increase in hemoglobin and red blood cell count. With a significant overdose, symptoms of cyanide poisoning appear: flu-like condition, drop in blood pressure, fever, dizziness, itching, weakness, nausea, and in severe cases, death.
Isolated cases of positive response to amygdalin treatment have been described in the literature. Even in those studies where amygdalin and its analogues were considered ineffective, about a quarter of the cases showed an improvement in the quality of life of patients, although many of them experienced adverse reactions and symptoms of cyanide poisoning *.
As with vitamin C, the evidence base for the benefit of amygdalin in breast cancer is still very weak * due to the lack of large, conclusive clinical studies *. The selectivity of its action against cancer cells, like vitamin C, is not large enough. And compared to vitamin C, amygdalin subjectively appears to be a less effective therapy. In addition, the therapeutic amygdalin (under the brand name Laetrile™) is produced primarily in Mexico and may be difficult to obtain.
Artemisinin, contained in the annual mugwort, also causes the death of cancer cells by increasing the concentration of oxidizing agents. But, unlike vitamin C and amygdalin, artemisinin causes the death of cancer cells not only through apoptosis or necrosis, but also through the so-called ferropoptosis *.
Inside cancer cells, especially in lysosomes, there is an abnormally high iron content, which is required for the cells to divide. Reacting with iron, artemisinin in large quantities forms various forms of free radicals that destroy protein molecules, including lysosome membranes. The breakthrough of the aggressive content of lysosomes into the cytosol of the cell produces catastrophic destruction inside it, leading to cell death *.
The specific nature of artemisinin's production of free radicals allows it to be combined with other therapies as a complementary treatment *. Preclinical studies show that artemisinin has a harmful effect only on tumor cells, but not on immune cells *. Unfortunately, artemisinin is poorly soluble in water and fats, has low oral bioavailability and a short elimination half-life. Other disadvantages are its side effects, including neurotoxicity, brachycardia, decreased hemoglobin levels, and others.
Several synthetic and semi-synthetic artemisin preparations have been developed that largely overcome these shortcomings, but clinical evidence for their effectiveness is still very weak. Anecdotal reports of clinical studies of artemisin compounds have not yet shown high results * * *. No reversal of the tumor process under the influence of artemisin preparations has been reported so far.
Salinomycin is an ionophore antibiotic used to treat coccidiosis in poultry. Salinomycin is considered a potentially promising antitumor agent, but there is not yet enough convincing data to recommend it for clinical approval.
Salinomycin exhibits its cytotoxic effect in vitro in several ways. First, it creates oxidative stress inside the cells through the production of reactive oxygen species (ROS), which leads to impaired mitochondrial function and subsequent apoptosis * *. Secondly, it activates the stress of the cell's endoplasmic reticulum, which causes the death of cancer cells by autophagy *. However, both of these actions can weaken each other. Autophagy attenuates apoptosis induced by ROS production, thereby protecting cancer cells * *.
Salinomycin is a powerful inhibitor of P-glycoprotein *, which provides cells with insensitivity to chemotherapy by actively removing toxins from the cell that have entered it. Moreover, salinomycin copes with this task more effectively than verapamil *.
While commonly used chemotherapy drugs target cancer cells in the tumor array, salinomycin selectively * kills breast cancer stem cells * as well as multidrug * and apoptotic * resistant cancer cells. Of particular note is that salinomycin is effective against triple negative breast cancer stem cells * *. Thus, the combination of salinomycin with traditional therapy seems promising, which could reduce the risk of recurrence of the disease.
The combination of salinomycin and almost any standard cytotoxic agent enhances their effect, albeit with varying degrees of severity *. The combination of salinomycin with trastuzumab (an anti-HER2 monoclonal antibody) also works synergistically *. Salinomycin increases the sensitivity of luminal type A cancer cells to tamoxifen, preventing them from developing drug resistance * *. In addition, salinomycin aggravates the sensitivity of cancer cells not only to chemotherapy, but also to radiation therapy *.
In mouse models, compared to controls, intraperitoneal injections of salinomycin (2.5 mg/kg) reduced the growth of the implanted tumor by an order of magnitude *, causing apoptosis, necrosis, and differentiation of epithelial cancer cells. Daily intraperitoneal injections of salinomycin (5 mg/kg) or paclitaxel (5 mg/kg) for 5 weeks in mammary tumor-grafted mice (SUM159) delayed the formation of a palpable tumor by 2 weeks compared to control animals. After 4 weeks of treatment, the number of cells forming mammospheres in the salinomycin group was half as much. While paclitaxel led to a 2-fold increase in metastasis formation, salinomycin, on the contrary, led to a 4-fold decrease *. Since mammospheres are primarily composed of cancer stem cells *, this effect suggests that salinomycin is capable of destroying them.
In patients with metastatic breast, ovarian, and head and neck cancers, intravenous salinomycin resulted in partial regression of tumor metastasis with few acute and long-term side effects *. Here, salinomycin (200-250 µg/kg every other day for three weeks) was administered both alone and in combination with erlotinib, enhancing the effect of the latter. But this seems to be the only known clinical study of salinomycin to date, conducted back in 2012.
Salinomycin, however, is highly toxic and can damage nerve cells and normal stem cells, limiting its clinical use. Acute poisoning in humans can already be caused by a dosage of 1 mg/kg *. Typical symptoms are shortness of breath, dizziness, nausea, weakness in the legs, photophobia and high blood pressure *. However, exacerbation of sensitivity to antimitotic drugs can be achieved at very low concentrations of salinomycin, allowing combinations with chemotherapy at doses of salinomycin that the patient is able to tolerate.
Proliferation suppression. While cytotoxins directly kill cancer cells, antimitotic agents prevent them from dividing by delaying the cell cycle. Antimitotic substances can have different effects on tubulin * and act at different stages of the cell cycle *.
Antimitotic phytopreparations, just like standard chemotherapy drugs, are used with rapid tumor growth, and for no more than 1.5 months. For the duration of chemotherapy, all of the above items of the program †, except for diet and general tone, are canceled. Women of childbearing age should use effective methods of preventing pregnancy at least one month before the start of a course of chemotherapy, as well as during the course, and within a month after its completion.
Quite a lot of natural substances are known to have an antimitotic effect *, although much weaker than standard chemotherapy drugs, including those developed on their basis. However, the evidence for their antimitotic activity is based largely on in vitro experiments, and few of them have been clinically tested.
Hormone independent breast cancer cells (MDA-MB-231). Of 897 natural product ethanol extracts tested in vitro *, toxicity-independent 50% growth inhibition (IG50) below 30 µg/mL was shown by 14, of which only the following 7 are available *:
Mandrake (Podophyllum peltatum), root, (IG50 < 1 µg/mL);
Thuja (Thuja occidentalis), twigs with leaves (IG50=4.4 µg/mL);
White mistletoe (Viscum album), leaves (IG50=9.6 µg/mL);
Tou gu cao (Speranskia tuberculata), grass (IG50=14.0 µg/mL);
Bentonite clay (IG50=14.7 µg/mL);
Madder (Rubia tinctorum), root (IG50=20.5 µg/mL);
Elecampane (Inula helenium), root (IG50=28.8 µg/mL).
Other available extracts are less effective:
Terminalia (Terminalia arjuna), stem bark (IG50=40.8 µg/mL);
Pomegranate (Punica granatum), fruits (IG50=41.0 µg/mL);
Juniper (Juniper Communis), berries (IG50=42.0 µg/mL);
Boswellia (Boswellia carterii), resinous frankincense (IG50=58.8 µg/mL);
Eucalyptus (Eucalyptus globulus), leaf (IG50=59.0 µg/mL);
Stonebreaker (Phyllanthus niruri), leaf (IG50=59.6μg/mL),
Wild geranium (Geranium maculatum), root (IG50=60.2 µg/mL);
Rhodiola Kirillova (Rhodiola kirilowii), root (IG50=64.7 µg/mL);
Umbellate wintergreen (Chimaphila umbellata), aerial part (IG50=66.2 µg/mL);
Clove (Syzygium aromaticum), flower buds (IG50=68.3μg/mL);
Fish mint (Houttuynia cordata), root (IG50=72.1 µg/mL);
Japanese knotweed (Polygonum cuspidatum), root (IG50=73.4 µg/mL);
Blackberry (Rubus fruticosus), fruits (IG50=74.3 µg/mL);
Madder (Rubia cordifolia), root (IG50=76.6 µg/mL);
Neem (Azadirachta indica), leaves (IG50=79.4 µg/mL);
Maiden tansy (Tanacetum parthenium), flowers (IG50=80.1 µg/mL).
The extracts were prepared at the rate of 5 g of raw material dry powder per 100 ml of absolute ethanol.
Some plant extracts in vitro are able to stop the division of tumor cells only due to their high toxicity:
Wild yam (Dioscorea villosa), root, (IG50=0.1 µg/mL);
Wake robin (Trillium pendulum), root (IG50=1.1 µg/mL);
Hoary puccoon (Lithospermum canescens), root (IG50=4.6 µg/mL);
Drago (Dracaena draco, Croton draco), leaf and bark pigment (IG50=12 µg/mL);
Sweet flag (Acorus calamus), root (IC50=13.7 µg/mL *).
Of these five plants, only the first and last are available.
Preclinical studies show apoptotic, antiproliferative and antimetastatic effects also of the following plants:
Sophora (Sophora flavescens), root * * *;
Ginkgo biloba (Ginkgo biloba), leaves * *;
Canadian goldenseal (Hydrastis canadensis), whole plant, root *;
Aloe vera (Aloe vera), leaves * *.
Hormone dependent breast cancer cells (MCF-7). Among the 76 studied chloroform-ethanol extracts of plants *, the highest antiproliferative effect in vitro was shown by:
Inula (Inula graveolens), root (IC50=3.83 μg/mL),
Dominican sage (Salvia dominica), leaves (IC50=7.28 μg/mL),
Canadian horseweed (Conyza canadensis), root (IC50=12.76 μg/mL),
Jordan yarrow (Achillea santolina), flowers (IC50=24.12 μg/mL).
Other antiproliferative plants:
Evaluation of medicinal plant extracts * used in traditional Indian medicine for cancer treatment showed significant antioxidant and antiproliferative activity of 10 of them (in descending order of activity): Tropical milkweed (Asclepias curassavica) → Bermuda grass (Cynodon dactylon) → Costus (Costus speciosus) → Amarant (Amaranthus tricolor) → Woodroses (Merremia emerginata) → Ophiorrhiza (Ophiorrhiza mungos) → (Tabernaemontana heyneana) → (Blepharis maderaspatensis) → Bael (Aegle marmelos) → Chaff-flower (Achyranthes aspera). Amaranth red (Amaranthus paniculatus) or Amaranth tricolor (Amaranthus tricolor), is widely distributed in Europe. Amaranth leaf ethyl acetate extract has an IC50 of 19.21 µg/mL for the MCF-7 cancer cell line versus 39.44 µg/mL for the non-cancer VERO cell line after 72 hours of in vitro treatment.
In another in vitro study, more than 150 traditional plants associated with estrogenic effects markedly inhibited the growth of ER– breast cancer cells Mandrake (Podophyllum peltatum), Bloodroot (Sanguinaria canadensis), Juniper (Juniperus communis), and Mistletoe (Viscum album) *.
Due to the lack of knowledge, inaccessibility, difficulty of normalization and assimilation, delivery problems, rapid removal from the body and the non-selective effect of the active substances of these plants, only a few of this list are of practical interest *.
• White mistletoe (Viscum album).
The antitumor activity of mistletoe in vitro has been demonstrated through various mechanisms: induction of apoptosis and necrosis, inhibition of the cell cycle * and activation of the specific and non-specific immune system *. The combination of mistletoe leaf extract with trastuzumab enhanced the in vitro anticancer effect of the latter against the HER2+ breast cancer cell line (SK-BR-3) *. Similar synergistic effect of inhibition of proliferation of both ER+ (MCF-7) and ER– (MDA-MB-231) cell lines *.
Several clinical trials of breast cancer patients treated with mistletoe chemotherapy have reported improvements in survival, tumor size, remission, quality of life, and reduced side effects of chemotherapy * * *. The safety and efficacy of mistletoe has been established in a multicenter clinical study *.
• Wild yam (Dioscorea villosa).
In vitro studies show a strong anti-cancer effect of wild yam root ethyl extract and its various components *. The extract significantly reduces the viability of ER+ (MCF-7) and ER– (MDA-MB 231) cancer cells at a concentration of 50 µg/mL by enhancing DNA methylation *.
Dioscin exerts its effects through the regulation of a large number of genes *. In the presence of 5.76 µM dioscin, invasion of TNBC cells (MDA-MB-231) was reduced by 65 *.
Dioscorealide B showed a cytotoxic effect against ER+ MCF-7 cells (IC50=2.76 μM) and TNBC MDA-MB-468 cells (IC50=9.93 μM) *.
Diosgenin, a steroidal sapogenin considered to be a phytoestrogen, causes marked inhibition of MDA-MB-231 * cell migration and suppresses fatty acid synthase expression in HER2 * cells.
Diosgenin can effectively inhibit the in vitro stem properties of CSCs and induce apoptosis in breast cancer cell lines such as ER+ (MCF-7, T47D) and ER– (MDA-MB-231) breast cancer cells *.
Due to the fact that diosgenin is a hydrophobic substance, fatty solutions give higher and more stable plasma levels than aqueous solution. In a clinical study of healthy volunteers, the oral dose was 50 mg/day of yam extract in olive oil solution, corresponding to 8 mg/day of diosgenin, or 2'000 mg/day of dry yam root *. As a result of taking diosgenin supplements, cognitive functions were improved.
Meanwhile, the use of whole yam root extract in breast cancer still raises some concerns due to its possible estrogenic effects, and requires further clinical study.
In one study, ingestion of wild yam kept salivary estradiol levels at a consistently very low level, suggesting that the herb may inhibit estradiol synthesis *. In another study, postmenopausal women who replaced rice with yams (390 g/day) in their diets increased their serum estrone concentration by 26% within 30 days; sex hormone-binding globulin (SHBG) – by 9.5% and estradiol – by 27% *.
• Ashwagandha (Withania somnifera).
The plant contains withaferin A, which in ERα-positive tumors (MCF-7 and T47D) exhibits in vitro (2.5 µM) cytotoxic efficacy comparable to doxorubicin *. Mice treated with intraperitoneal injections of withaferin (4 mg/kg) had a significant delay in the development of both ER+ and ER– grafted tumors *. The daily dosage of Vitaferin, corresponding to a person, will be 25 mg.
• Elecampane (Inula helenium).
Elecampane contains a rich set of sesquiterpenoids. Sesquiterpene lactones in vitro suppress the expression of STAT3 target genes including cyclin D1, c-myc and bcl-2 and induce caspase-mediated apoptosis *. Isoalantolactone (20 µM) in vitro inhibits adhesion, migration and invasion of ER– MDA-MB-231 cells *.
• Sage (Salvia).
Ethanol extracts of Salvia syriaca, Salvia fruticosa, Salvia horminum demonstrated in vitro selective antiproliferative activity against ER+ breast cancer cell lines MCF-7 with minimal toxicity against normal fibroblasts *, extracts of Salvia triloba and Salvia dominica also against ER+ cells T47D *, and extracts of Salvia miltiorrhiza exhibits strong antiproliferative effects on ER+ cells MCF-7, causing G1 cell cycle arrest via Akt and p27 mechanisms *.
Tanshinone IIA – the active substance of sage, in vitro inhibits proliferation and induces apoptosis of cancer cells by suppressing multiple genes involved in the regulation of the cell cycle, proliferation, cell apoptosis, and DNA synthesis. When treated with tanshinon, the proliferation of breast cancer cells was significantly suppressed (IC50=0.25 μg/mL). Moreover, the number of apoptotic both ER+ and ER– cells increased, while tamoxifen inhibited only ER+ cells *. Tanshinone reduces the expression of ABC transporters, including P-gp, BCRP and MRP1, which pump chemotherapy drugs out of the cell *. After treatment with tanshinon, cell proliferation and formation of the CSC mammosphere were significantly reduced; and growth and mean tumor weight were significantly reduced. Tanshinon has the potential to kill CSCs and may interfere with their growth both in vitro and in vivo *.
• Licorice (Glycyrrhiza glabra).
Glabridin, one of the components of licorice root, attenuates tumor growth, invasion, migration, and CSC-like properties of ER– mice inoculated mammary tumor (MDA-MB-231) * *. An alcoholic extract of the flavonoid complex of licorice root (100 mg/kg) halved the growth of the mass of a ER– breast tumor (MDA-MB-231) inoculated into mice with suppression of inflammation, blocking of iNOS expression, and inactivation of the JAK2/STAT3 * protumor signaling pathway. However, in human terms, this dosage of the extract would be 500 mg/day, which would require about 200 g of dry raw materials *, which is clearly higher than the recommended prescription dosage. At the same time, low doses of the extract may, on the contrary, have an estrogenic and proliferative effect on ER+ cells (MCF-7) *.
• Baikal skullcap (Scutellaria baicalensis).
Scutellarin, wogonin, baicalein and baicalin are considered to be the main medicinal components of the aerial parts of skullcap. In order to achieve the separation of baicalin and baicalein, water or methanol is used as a solvent to extract the former, and acetone or ethyl acetate is used for the latter.
Wogonin given orally for 4 weeks at a dose of 10 mg/kg reduced the growth of grafted ER+ (T47D) tumors by up to 88% and ER– tumors (MDA-MB-231) by up to 48% without toxicity *. In osteosarcoma, in vitro wogonin (40-80 µM) reduces CSC renewal opportunities by inhibiting sphere formation and effectively minimizes the potential risk from CSC *.
Baicalein inhibits proliferation, migration, and invasion of MDA-MB-231 cells in a time- and dose-dependent manner. In MDA-MB-231 mammary tumor-grafted mice, baicalein (100 mg/kg oral) was effective in reducing the rate of metastasis and the size of the metastatic lesion *.
Baicalin is able to suppress the invasion and migration of breast cancer cells in vitro, without affecting, however, their viability *. Mice inoculated with TNBC cancer cells (MDA-MB-231) treated with intraperitoneal injections of baicalin (100 mg/kg) halved the number of tumor formations compared to control mice. The sizes of tumor nodules were also smaller, and the weight of the tumor was 4 times lower *. However, scutellarin and baicalin may exhibit estrogenic and proliferative effects in ER+ cells (MCF-7), while wogonin and baicalein (50 µM) inhibit the growth of these cells *.
The whole aqueous extract of skullcap inhibits both glycolysis and oxidative phosphorylation in tumor cells *. In one clinical study, 3 out of 27 patients with metastatic breast cancer experienced regression of the primary tumor *. In another similar clinical trial, patients took 350 ml of a standardized skullcap extract daily. Of the 21 participants, 7 stabilized and 5 regressed to varying degrees *.
• Feverfew (Tanacetum parthenium).
Parthenolide, the active substance of the plant, in vitro inhibits the formation of the MCF-7 mammosphere, as well as the proliferation and formation of colonies of the MCF-7 population. These effects resulted from the inhibition of NF-κB activity in MCF-7 cells grown both as 2D cultures and as mammospheres *. Parthenolide and preparations based on it increase oxidative stress in cancer cells, suppress mitochondrial respiration and increase the expression of RIP-1, which led to the death of cancer cells by necrosis.
Despite its high in vitro efficacy, the therapeutic use of parthenolide is difficult due to its poor solubility. To solve this problem, an alcohol-soluble dimethylamino analogue of parthenolide (DMAPT) was created. Administration of DMAPT (50 mg/kg oral) to nude mice with xenografts of MDA-MB-231 cells resulted in significant inhibition of tumor growth, increased survival of the animals, and a marked reduction in lung metastasis *.
• Broccoli (Brassica oleracea).
Compared to other plants, broccoli contains the highest concentration of indole-3-carbinol (I3C). I3C enhances the binding of nucleostemin (a breast cancer stem cell surface marker) to a tumor suppressor inhibitor that interacts with the p53 protein, causing p53 to be released and induce the cancer cell to apoptosis *. A concentration of 200 μM I3C, due to its metabolite 1-benzyl-I3C (1-benzyl-I3C), almost completely prevents the formation of tumor mammospheres in vitro. To achieve this concentration, mice grafted with a stem cell-enriched mammary tumor (10AT-HER2) were given subcutaneous injections of I3C at a dosage of 300 mg/kg. In human terms, this would be 25mg/kg or about 2'000mg/day, while in clinical trials I3C dosage did not exceed 1'200mg/day *, and the maximum effect of improving estrogen metabolism due to I3C was observed at 400mg/day *.
• Garlic (Allium sativum).
Diallyl disulfide (DADS), an oil-soluble organosulfide found in garlic root, attenuates the protumor effect of linoleic acid and enhances the antitumor effect of eicosapentaenoic acid in ER– MDA-MB-231 cells. In mice inoculated with ER+ mammary tumor (KPL-1), intraperitoneal injections of DADS (1-2 mg) thrice weekly for 35 days caused growth retardation and a 43% reduction in primary tumor mass compared to untreated animals, showing no no visible side effects *.
• Amaranth (Amaranthus tricolor).
Amaranth leaves in vitro effectively suppress the pro-inflammatory enzymes COX-1 and COX-2, and exhibit antiproliferative activity against colon, breast, lung, and stomach tumor cell lines *. Amaranth leaves at a dosage equivalent to a human dose of 1'300 mg/day showed an anti-proliferative effect comparable to that of 5-fluorouracil in two-week trials in mice inoculated with Ehrlich's carcinoma *.
The need to focus on cancer stem cells further reduces the recommended list * *. Among the plants selected above that were successful against tumor array cancer cells, only the following were successful against CSC in vitro: wild yam, fenugreek (active ingredient – diosgenin); licorice (active ingredient – glabridin); tansy (active ingredient – parthenolide); broccoli (active ingredient – indole-3-carbinol), sage (active ingredient – tanshinon), skullcap (active ingredient – baicalein).
However, whatever success all of these plants show in contact with cancer cells in two-dimensional in vitro studies, clinical trials may show many times less modest results.
Decreased cellular energy. One of the hallmarks of a cancer cell in most cases is a modification of its metabolism.
As cells malignantly transform, the following characteristics appear related to their internal energy:
- The number and/or activity of mitochondria decreases. Accordingly, the function of oxidative phosphorylation (OP) decreases.
- On the other hand, glycolysis and fermentation, a less efficient way of producing energy compared to RP, is increased, resulting in limited energy production and reduced thermal energy.
- The intracellular level of Na+ ions increases relative to the extracellular level of K+ ions. This reduces the efficiency of membrane K/Na pumps that affect cell communication and cell membrane dynamics * *.
All of the above processes lead to a decrease in the internal energy of transforming and cancer cells. The transmembrane potential of such cells can be significantly weakened, which will incline them to division *. A decrease in the electric field strength of the cell membrane, in turn, causes changes in the metabolic functions of the cell. The negatively charged field around the cell will repel other negatively charged cells such as red blood cells and lymphocytes and will not allow oxygen and the immune system to destroy cancer cells *.
On this basis, it is logical to assume, and the results of various experiments confirm this, that the restriction of energy production hits normal cells less hard than cancer cells, including CSC. Therefore, many strategies that interfere with energy metabolism can be used as part of multitarget anti-cancer therapy *.
Techniques for limiting cell energy have already been discussed in the «Metabolic Correction» section †. Here we will only repeat some points:
Decreased glucose intake. The smartest suggestion would be to restrict calories, and especially simple sugars, which can create high blood glucose levels. In addition, you can consider the possibility of using substances that impair the absorption of carbohydrates (metformin, silibinin, apigenin, quercetin), reduce the production of glucose in the liver (metformin), block glucose transporters into cells (genistein, quercetin, kaempferol, diclofenac), suppress the accumulation of glucose in cells (citrus essential oils).
Suppression of glycolysis. Breast cancer cells are characterized by a strong dependence on glycolytic metabolism. Glycolysis makes it possible to generate energy in the hypoxic environment in which tumor cells reside. Thanks to glycolysis, the intracellular production of building material for the creation of daughter cancer cells is increased, and the necessary microenvironment is created for their invasion and metastasis. Glycolytic metabolism supports stem cells in several types of cancer *. Thus, inhibition of glycolysis can significantly increase the effectiveness of antitumor therapy.
Glycolysis inhibitors reduce the energy capacity of cancer cells, as well as the intracellular production of building material to create a new cell.
Limitation of mitochondrial respiration. Mitochondria not only provide economical production of cellular energy. They play a vital role in cellular processes such as cell signaling, metabolism, regulation of growth and regulation of cell death, i.e. processes that directly affect the tumor process. Including regulation of stem and cancer cells, their identity, differentiation and choice between life and death.
Although breast cancer cells are characterized by a high level of anaerobic respiration, when aerobic respiration is suppressed, they show a weakening of the ability to grow and an increase in sensitivity to cytotoxic drugs *. Therefore, the control of mitochondrial functions is a promising direction in oncology. Nevertheless, given the exceptional importance of mitochondria in cell life, the suppression of their functions cannot be long-term, and is more suitable only during a course of antitumor therapy.
The process of oxidative phosphorylation can be disrupted by electron transport chain inhibitors such as menadione and salinomycin.
It is important to note that cancer cell subpopulations (MCF-7) with the highest mitochondrial levels of oxidants and mitochondrial biogenesis have the highest ability to efficiently form mammospheres *. However, mitochondrial biogenesis and translation can be inhibited by some antibiotics such as doxycycline, azithromycin, tigecycline, pervinium pamoate *. These antibiotics inhibit protein synthesis by acting on either the large or small subunit of ribosomes *, while pyrivinium pamoate inhibits the process of oxidative phosphorylation *. This makes it possible to repeatedly reduce the viability of tumor-initiating cells.
Combined methods. Because cells can switch from one resource to another for energy production, inhibition of glycolysis alone or mitondrial metabolism alone is likely to be less effective than a combination of the two.
Thus, doxycycline and high-dose vitamin C create a lethal combination targeting both mitochondrial and glycolytic metabolism that ultimately destroys the CSC *. Suppression of mitochondrial function by doxycycline forces cancer cells to switch to glycolytic respiration. After that, glycolysis-blocking vitamin C overdoses leave them on a starvation diet. And oxidants, which are massively formed as a result of the activity of vitamin C inside the cell, act on them much more destructively than on normal cells. An alternative to vitamin C in this case may be berberine and parthenolide *.
Even more effective is the addition of azithromycin to doxycycline and vitamin C. The combination of low doses of doxycycline and azithromycin 10-fold reduces the ability of CSCs to form mammospheres by inhibiting ribosomes. Whereas doxycycline inhibits the small mitochondrial ribosome and azithromycin inhibits the large mitochondrial ribosome, ultra-high doses of vitamin C exhibit a pro-oxidant effect.
The ascorbate radical is usually quite stable, but in the presence of metal ions it becomes very reactive. Due to the high content of iron in cancer cells, mitochondria become a sensitive target of its prooxidant effect. Ascorbate-induced oxidative stress stimulates mitochondrial biogenesis, which requires increased mitochondrial metabolism and ATP production. As a result, the mitochondrial functions of CSC are almost completely switched off, and ATP is significantly depleted, which leads to their death *. All three components of this combination have a higher safety profile than most standard chemotherapeutic agents, as well as many years of clinical experience and a very low price.
Suppression of invasion and metastasis. Metastasis is the process during which cancer cells pass through the so-called epithelial-mesenchymal transition (EMT), as a result of which they acquire high mobility and enter the blood and lymphatic systems. Then they spread to distant organs and tissues of the body, where they undergo a reverse transformation – the mesenchymal-epithelial transition (MET), as a result of which they lose their mobility and subsequently begin their growth in a new place.
• Metformin attenuates the expression of genes involved in the remodeling of the extracellular matrix of tumor tissues, impairs EMT and, in general, reduces the overall level of metastasis in experimental animals *. Equivalent dosage: 3×500 mg.
• Piperine, an alkaloid from black pepper (Piper nigrum), significantly reduced the expression of MMP-2/9 * and MMP-13, and inhibited the migration of 4T1 cells in vitro *. Piperine inhibits in vitro growth of TNBC cells and hormone-dependent breast cancer cells, and also enhances the effect of radiation therapy without affecting the normal growth of breast epithelial cells *. Piperine injections (5 mg/kg for a month on alternate days) doubled the growth of the primary mouse mammary tumor and also significantly inhibited lung metastasis *.
• Zoledronic acid, a third-generation bisphosphonate, in vitro (10 μM) reduces not only proliferation, but also the expression of mesenchymal markers, and also increases the expression of E-cadherin in TNBC cells *.
• Fisetin is a flavonol which is especially rich in onions, tomatoes, cucumbers, apples, grapes, strawberries, mangoes and persimmons *. It interferes with key regulators of EMT and converts it into MET, thereby inhibiting metastasis. Fisetin in vitro (100 μM) inhibits cell proliferation, migration and invasion in TNBC cells (MDA-MB-231 and BT549), and subcutaneous injections of fisetin at a dose of 100 mg/kg for 3 weeks of the experiment halved the development of primary TNBC tumor (MDA- MB-231) in mice *. Fisetin in vitro (100 μM) inhibits cell proliferation, migration and invasion in TNBC cells (MDA-MB-231 and BT549), and subcutaneous injections of fisetin at a dosage of 100 mg/kg over a 3-week experiment halved the development of primary TNBC tumors in mice *.
• Quercetin is a flavonol found in grains, onions, apples, and other fruits and vegetables. Intraperitoneal injections of quercetin (50 mg/kg) reduced melanoma lung metastasis in mice *, and intravenous administration of quercetin or its isoform, pterostilbene (20 mg/kg), reduced the growth of liver metastasis in mice by 73% compared to control *.
• Resveratrol is stilbene, which is found in large quantities in the skin of dark grapes. Orally administered (100 mg/kg for 21 days), resveratrol significantly reduced cell adhesion, migration, invasion, and MMP-9 activity of grafted mouse tumor cells in mice. The number of lung metastases was also reduced *. Oral administration of resveratrol (50 mg/kg) for 17 days inhibited primary tumor growth by 60% compared to controls and also inhibited lung metastases in melanoma-grafted mice *.
• Silymarin is flavonolignans from milk thistle seeds, showing in vitro antimetastatic effects in various types of cancer *. Silymarin has at least 8 main components, including 7 flavonolignans: silybin A, silybin B, isosilybin A, isosilybin B, silychristin, isosilichristin, silydianin, and also one flavonoid – taxifolin. Since flavonolignans are poorly soluble in water, their conjugates with phosphadylcholine (eg Siliphos®) have been developed. A daily three-week intake (450 mg/kg) of a complex of silybin with phosphatidylcholine (silipid) delayed the development of spontaneous mammary tumors in transgenic mice and reduced the number of mammary tumors *. The complex significantly reduced the proportion of mice with lung metastases, as well as the average size of metastases, although in another study, dietary intake of silibinin did not have a noticeable effect on tumor growth *. Dosage of silibinin: 1'400 mg/day *.
• Salinomycin, an ionophore antibiotic used in veterinary medicine, alters the expression of genes involved in metastasis, tumor formation ability and EMT differentiation * * *. Combinations of salinomycin with conventional chemotherapy drugs (doxorubicin or paclitaxel), with anti-HER2 therapies (trastuzumab or lapatinib), with resveratrol, as well as with histone deacetylase inhibitors, synergistically suppress the growth of the primary tumor * * * *. In vitro, salinomycin alone kills breast CSCs 100 times more effectively than the standard chemotherapy drug paclitaxel *. Some modified salinomycin analogues were even more effective than salinomycin in inhibiting TNBC cell migration (MDA-MB-231) *. Many migration-related parameters were significantly lower in MDA-MB-231 cells after salinomycin treatment than in control cells *.
• Diallyl trisulfide (DATS) in vitro (10 µM) inhibits migration and invasion of TNBC cells (MDA-MB-231 and HS578t) by inhibiting MMP2/9 metalloproteinases and NF-κB and ERK/MAPK signaling pathways *. Daily dose: 2 cloves, i.e. 10 g raw garlic, equivalent to 10 mg DATS *. The use of dry garlic (3-4 tbsp per day) allows you to significantly increase the intake of DATS without emitting a garlic smell.
• Ellagic acid, luteolin and punic acid contained in pomegranate juice in vitro not only inhibit tumor growth, but also reduce the migration of cancer cells, both estrogen-positive (MCF-7) and estrogen-negative (MDA-MB-231) of tumors without affecting the behavior of normal cells *.
• Curcumin is curcuminoid from the root of turmeric. Curcumin inhibits in vitro (15 μM) migration by restoring the efficacy of E-cadherin, a tumor suppressor that is highly downregulated in breast cancer cells *, and reduces the expression of MMP-2/9 metalloproteinases *.
• Lycorine is a natural alkaloid found in the flowers and bulbs of Amaryllidaceae species such as daffodils (Narcisseae), snowdrops (Galantheae) and lycoris (Lycoreae) *. It is a strong inducer of apoptosis and can induce both mitochondrial and death receptor-mediated apoptosis in cancer cells. Lycorine has a moderate anti-cancer effect against various malignant tumors *, including breast cancer, with low toxicity *. Intraperitoneal administration of lycorine significantly delayed the growth of mammary tumors in experimental mice.
• Anticoagulants such as aspirin reduce the chances of clotting, and proteolytic enzymes such as bromelain safely unmask the platelet lining of migrating cancer cells.
• Antihistamines show ambiguous action. A large retrospective study showed that long-term use of H1 antihistamines such as ebastine, loratadine or desloratadine, significantly increases the survival of patients with breast cancer of both ER+ and ER– subtypes, while cetirizine and clemastine act in the opposite direction *.
Suppression of angiogenesis. Angiogenesis is regulated by a balance between pro- and anti-angiogenic factors.
A key angiogenic factor, vascular endothelial growth factor (VEGF), is regulated by estrogen in both normal * * and cancerous breast tissue * *. Another powerful angiogenic factor is angiogenin, which induces endothelial cell proliferation after its nuclear translocation * *. Nuclear translocation of angiogenin is also required for the angiogenic response of VEGF and other angiogenic regulators *.
Angiogenin is also regulated by estrogen, as is VEGF * * *. On the other hand, they are opposed by endostatin, one of the most important antiangiogenic regulators. Endostatin inhibits the proliferation and migration of endothelial cells and induces apoptosis of proliferating endothelial cells * * *. Endostatin can also regulate the VEGF/VEGFR signaling pathway by direct action on tumor tissue cells or upregulate other anti-angiogenic genes *. Estradiol reduces and tamoxifen increases the production of endostatin * * *.
Some natural remedies can be used to suppress angiogenesis:
• The combination of flaxseed and tamoxifen has been evaluated in clinical studies * *. Although it was not possible to identify the active substance and its mechanism, it was found that ground flaxseed (25 g/day) did not reduce the levels of VEGF or angiogenin, but, like tamoxifen, significantly increased the concentration of endostatin in breast tissue.
• Genistein enhances breast cancer cell adhesion by reducing cell migration * and at 15 mg/kg reduces angiogenesis in mice *. The addition of 750 mg of genistein to 1 kg of food reduces the number of metastases in mice by 10 times after surgical removal of a mammary tumor *.
• Curcumin in vitro reduces the activity of MMP-9 * and MMP-2 * proteinases, inhibits growth factor receptors such as EGFR and VEGFR. Curcumin administered intraperitoneally (300 mg/kg) to mice inoculated with triple-negative breast cancer cells (MDA-MB-231) showed a significant decrease in the expression level of pro-angiogenic factors and a decrease in microvascular density in experimental animals in 21 days *. Intraperitoneal injections of curcumin (60 mg/kg) in mice with glioblastoma (U-87) implanted inside the skull reduced tumor volumes by 32% compared to controls *. At the same time, the enzymatic activity of MMP-9 decreased by 25%. Curcumin, at 2% of the food intake for 6 weeks, in AR+ tumor-grafted prostate (LNCaP) mice produced a marked decrease in the rate of cell proliferation due to apoptosis, as well as a significant decrease in microvascular density *.
• EGCG (epigallocatechin gallate) and other green tea polyphenols regulate the activity of a number of key enzymes, which leads to the blocking of endothelial cell proliferation and suppression of metastasis through urokinase and matrix metalloproteinases. Green tea polyphenols reduce angiogenesis, in particular by reducing vascular endothelial growth factor and receptor phosphorylation *.
• Ellagitannins. Pomegranate extract enriched to 37% ellagitannins and 3.5% ellagic acid can nearly halve tumor-associated angiogenesis in prostate tumor-grafted mice (LAPC4) by oral administration (0.8 mg/day, human equivalent of 380 ml/day of pomegranate juice) *.
Intraperitoneal injections of ellagic acid (100 mg/kg for 25 days) decreased the growth rate of the grafted TNBC breast tumor (MDA-MB-231) by 6 times in mice *.
Daily consumption of 1 glass of pomegranate juice can lengthen the doubling time of a prostate tumor in men from 15 to 54 months *. Interestingly, the inhibitory effect appears in hypoxic but not normoxic tissue. Note that ellagitannins themselves are not absorbed into the body, but enter the bloodstream in the form of ellagic acid, into which they are hydrolyzed under the action of intestinal microflora. A 1:4 combination of ellagic acid and phosphatidylcholine can double its bioavailability *.
• Proanthocyanidins from grape seed (20 µg/mL) delay in vitro invasion of head and neck squamous cell carcinoma by reducing EGFR expression and reversing the epithelial-mesenchymal transition *. In prostate tumor-grafted (DU145) mice given 100-200 mg/kg orally of grape seed extract (Traconol™) for 7 weeks, tumor proliferation fell by two-thirds, apoptosis increased 3-4-fold, and the same the number of CD31-positive cells, which are a marker of angiogenesis, decreased *. Grape seed extract containing at least 85% procyanidins, and administered orally to mice (equiv. 650 mg/day) for 3 weeks before implantation of a breast tumor (MD-MBA-231), reduced the rate of its subsequent growth by 3 times *.
• Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). A decrease in the ratio of omega-6 to omega-3 fatty acids demonstrates a marked specific anti-angiogenic activity.
The ratio of copper to zinc is also important. Most cancer patients are deficient in zinc and have an excess of copper, and excess copper stimulates angiogenesis in the tumor.
• Several plants have traditionally been used as anti-angiogenic: Sweet wormwood (Artemisia annua), herb; European mistletoe (Viscum album), leaves and fruits; Turmeric (Curcuma longa), root; Baikal skullcap (Scullellaria baicalensis), root; Tea (Camellia sinensis), unfermented leaves; Ginkgo (Ginkgo biloba), leaves; Ginger (Zingiber officinale), root; Ginseng (Panax ginseng), root; grape seed extract (resveratrol and proanthocyanidin) * *.
However, the natural substances listed above seem to exhibit an indirect anti-angiogenic effect – it is associated with their other antitumor effects. A decrease in tumor activity will require less nutrients, which means that the tumor will need less blood vessels to develop to it.
Specific anti-angiogenic therapy, initially considered a promising direction, is currently being seriously questioned. Clinical observations in recent years show that anti-angiogenic therapy can be effective only together with chemotherapy, but not alone. The process of angiogenesis itself is not an independent phenomenon, it is a regulatory response to a lack of cellular nutrition. Based on mouse models, the researchers concluded that a lack of blood supply to a tumor makes it more invasive and more metastatic, while a tumor with sufficient blood supply is quieter. Thus, the overall effect of monotherapy with strong anti-angiogenic drugs, such as bevacizumab, may be the opposite of what is expected * *.
Suppression of stem characteristics
The saturation of a tumor with cancer stem cells is associated with its phenotypic features. Per 1'000 tumor cells, the number of CSC cells is: 1.1 in luminal subtype A (ER+/PR+, HЕR2–); 1.3 in luminal subtype B (ER+/PR+, HЕR2+); 8.6 for HER2+ subtype (ER–/PR–, HЕR2+); and above 17.7 in triple negative breast cancer subtype *. It is strikingly noticeable that the higher the concentration of CSC in the tumor, the more aggressive it is, and the worse the prognosis for the development of the disease.
Despite the fact that traditional chemotherapy drugs destroy the bulk of cancerous tumor cells, they trigger processes that help strengthen CSC * *. The surviving tumor cells after therapy, also known as the side population (SP), adapt to new conditions of existence, change their phenotype, develop the ability to effectively pump out chemotherapy drugs from the cell, and divide, creating a tumor resistant to repeated chemotherapy *. If surviving cancer stem cells * after treatment are the cause of tumor recurrence *, then the success of treatment depends not only on getting rid of the primary cancer mass or metastasis, but also on targeting the treatment to CSC *.
Based on the specific properties of CSC *, several therapeutic targets * * * * * have been proposed:
- cell surface markers (CD44+/CD24–, integrins) and functional markers (aldehyde dehydrogenase * *) CSC;
- pathways of self-renewal and differentiation (Wnt/β-catenin, Hedgehog, Notch *);
- cytokines and inflammatory pathways (eg IL-6, IL-8, TNF-α/NF-κB);
- pathways of TGF-β and epithelial-mesenchymal transition (Twist and Snail);
- molecules involved in metastasis; primarily CXCR4 or its ligand CXCL12;
- growth factors, their receptors and co-receptors (such as neuropilin-1) and signaling components (such as tyrosine kinases);
- drug resistance provided by MDR/ABC transporters (such as ABCG2) and ALDH1.
CSCs show amazing resilience and resilience to a wide variety of life challenges. Because of this, monotherapy is unlikely to be successful, and for this reason, a comprehensive approach is needed for reliable clinical success.
Several drugs already in use in human and veterinary medicine that are capable of targeting CSCs have been identified through screening or other observations; these are salinomycin, etoposide, abamectin, nigericin, tesmilifen and others *. Among them are some classic drugs such as metformin * * *, tranilast * and thioridazine *, which have been used for decades to treat respectively metabolic, allergic and psychiatric diseases.
Many natural substances, although more weakly, can also interfere with signaling pathways critical for maintaining CSC or for driving the CSC phenotype. These are, first of all, curcumin, piperine, quercetin, pterostilbene, resveratrol, berberine, green tea catechins, sulforaphane, indole-3-carbinol, genistein, gingerol, vitamin E, vitamin D, retinoic acid, parthenolide, triptolide, shogaol, pterostilbene, isoliquiritigenin, cyclopamine, coselrol, glabridin, coenimbin and many others *.
Some Chinese medicines and their active ingredients have also been shown to be toxic against CSCs; it is berberine from the rhizomes of Coptis (Coptis chinensis); oxymatrine from Sophora root (Sophora flavescens); baicalein from skullcap root (Scutellaria baicalensis); bufalin from parotid poison and skin glands of toads; CAPE from propolis and others *. Thus, the use of appropriate natural extracts could, to a certain extent, affect the stem cell characteristics in particular, and tumor development in general.
Wnt/β-catenin are signaling proteins involved in the coordination of cell behavior in the body. They modulate embryonic growth and regulate cell migration, proliferation, differentiation and survival *. Abnormal stabilization of β-catenin is seen in more than 50% of breast cancers *. Wnt/β-catenin signaling contributes to genomic instability and resistance to DNA damage, and conferring CSC resistance to radiation * * and chemotherapy * *. The decrease in β-catenin significantly inhibits the tumorigenic capacity of TNBC cells both in vitro and in vivo, and also reduces the stem properties of cancer cells *. Thus, inhibitors of Wnt/β-catenin signaling prevent the formation of colonies and the mammosphere of breast cancer cells *.
• Salinomycin, a veterinary antibiotic, inhibits the Wnt/β-catenin *, Hedgehog and Notch * signaling pathway. Treatment with salinomycin results in loss of expression of CSC-associated genes associated with poor tumor prognosis *. Salinomycin is highly effective in eliminating CSCs both in vitro and in vivo * *. In TNBC-grafted mammary tumor (SUM159) mice, administration of salinomycin (5 mg/kg intraperitoneally for 5 weeks) delayed the formation of palpable tumors by 2 weeks compared to controls.
• Niclosamide *, an anthelmintic drug, inhibits the Wnt signaling pathway, without showing toxicity to non-tumor cells *. It has been shown in animal experiments to successfully inhibit the activity of stem-like breast cancer cells and prevent the formation of mammospheres of breast cancer cells * *. Niclosamide not only inhibits the Wnt/β-catenin, mTORC1, STAT3, NF-κB, and Notch signaling pathways, but also targets mitochondria in cancer cells, causing cell cycle arrest, growth inhibition, autophagy, and apoptosis *.
The oral dosage of niclosamide used in long-term animal studies is 4-30 g/day in human equivalent *. Unfortunately, niclosamide's poor aqueous solubility and poor oral bioavailability can result in a too wide range of serum concentrations. Although intraperitoneal administration of 20 mg/kg niclosamide suppressed the growth of mouse mammary tumor (4T1) in mice without detectable toxicity *, the consequences of its injection in humans have not yet been studied.
• Acetaminophen, aka paracetamol, a popular anti-inflammatory, antipyretic and analgesic drug, causes both in vitro and in vivo differentiation of breast cancer cells through inhibition of the Wnt/β-catenin signaling pathway. Subcutaneous administration of acetaminophen for 28 days markedly reduced the growth of grafted TNBC tumor cells (MDA-MB-231) in mice *. The dosage here was 600 mg/kg, which in human equivalent corresponds to 4'000 mg/day, i.e. the maximum allowable daily dose for adults.
• Sulforaphane (50 mg/kg intraperitoneally for 2 weeks) inhibited Wnt/β-catenin pathway activation, reduced the number and size of mammospheres, and more than halved the growth of ALDH+-grafted human breast tumors in mice *.
• Ivermectin, a well-known antiparasitic drug, is a strong blocker of the Wnt pathway at low doses *. Ivermectin also interacts with several other targets, including the multidrug resistance (MDR) protein, the Akt/mTOR pathway, and some epigenetic deregulators predominantly targeting CSC. In addition, ivermectin, at a very low dose that did not cause overt cytotoxicity, overcame the drug resistance of cancer cells to chemotherapy drugs by reducing the expression of P-glycoprotein *. The highest dose approved by the FDA is 200 μg/kg, but ivermectin is well tolerated by patients with no noticeable toxicity at doses 10 times higher *.
Like salinomycin, ivermectin suppresses the viability of CSC-rich cell populations (CD44+/CD24- and cells growing in spheroids) more strongly than the general cell population *, but is much less toxic than salinomycin. It is highly valuable that the antitumor activity of ivermectin in vitro and in vivo is achieved at concentrations that may be clinically achievable based on pharmacokinetic studies in humans *. But, unfortunately, no reports of clinical trials of ivermectin in relation to cancer have been found.
• Curcumin and piperine both separately and in combination, by attenuating Wnt signaling, inhibit in vitro self-renewal of breast cancer stem cells and mammosphere formation, without causing toxicity to normal differentiated cells, but do not affect cell differentiation *. The combination of both substances enhances the effect of one another. Wnt signaling was inhibited by both curcumin and piperine half at 5 µM and completely at 10 µM.
• Vitamin D3 and its analogs in vitro inhibit Wnt transmission in cancer cells by several mechanisms *. They reduce the number of CSCs in breast tumors * and also promote the differentiation of colon carcinoma cells by inhibiting β-catenin signaling *. Oral administration of a synthetic analogue of vitamin D3 (0.1 µg/kg for 36 days) halved the volume and weight growth of grafted basaloid mammary tumors in mice *.
• Resveratrol and pterostilbene significantly reduce the viability and formation of the mammosphere with subsequent triggering of apoptosis in cancer stem cells. Resveratrol (100 mg/kg intravenously), by suppressing the Wnt/β-catenin pathway, reduced the growth of the grafted mammary tumor (MCF-7 and SUM159) in mice by a third, and reduced the CSC population among tumor cells. The stem properties of the cancer cells decreased so much that after re-inoculation of the treated tumor cells in secondary mice, they caused only 1 tumor out of 6 inoculations against 6 tumors in the control group within 30 days *. Mice treated with oral resveratrol (22.4 mg/kg every other day for 4 weeks) showed a significant reduction in the growth of the grafted TNBC tumor (MDA-MB-231) *. In human terms, the dosages of these two trials are 625 mg by injection and 140 mg orally, respectively.
• Baicalein inhibits both Wnt/β-catenin and a special AT-rich binding protein (SATB1) *in vitro (50 µM) and in vivo. In mice with grafted mammary tumor (MDA-MB-231), oral administration of baicalein (100 mg/kg for 15 days) significantly reduced liver metastasis.
• EGCG suppresses Wnt signaling * and stem cell division in inflammatory breast cancer, their invasive phenotype and survival *. Injections of the EGCG solution (16.5 mg/kg intraperitoneally) markedly slowed down the growth of grafted tumor derived from stem-like cancer cells in mice. Quercetin enhances the effects of EGCG in vivo by increasing the oral bioavailability of tea polyphenols and attenuating their methylation *.
• Diallyl disulfide (DADS), a fat-soluble component of garlic, inhibits the growth and metastatic potential of triple negative breast cancer cells in vitro and in vivo by inactivating the β-catenin signaling pathway * *. Dosage: 2-10 g raw garlic *.
• Genistein in vitro attenuates β-catenin-mediated expression of Wnt target genes in breast epithelial cells and promotes cell differentiation *. A dietary supplement of genistein (250 mg per 1 kg of food) slowed down the development of mammary tumors in mice *. A meta-analysis of several studies showed that consumption of soy food after breast cancer diagnosis reduced mortality by 15% and recurrence by 21%, and these rates were little affected by menopausal status and tumor ER status *.
Notch is a membrane receptor that plays an important role in intercellular communication and cell behavior by regulating decisions about cell fate, proliferation, differentiation and apoptosis * *. This signaling pathway regulates self-renewal and differentiation of breast stem cells * *, and abnormally high expression of elements of the Notch pathway * * contributes to the development of tumors, including the breast. TNBC tumors (MDA-MB-231) have significantly higher Notch activity than ERα+ tumors (MCF-7 and T47D) or HER2+ tumors (SK-BR-3) *.
At the same time, different Notch isoforms can play different roles * and can either promote tumor development or suppress it. For example, Notch1 appears to play an oncogenic role in breast cancer *, while knocking out Notch2 significantly accelerates TNBC tumor growth *. The Notch4 isoform seems to be the most important for the formation of the mammosphere *. Inhibition of Notch4 signaling reduced mammary CSC and completely inhibited tumor initiation *. Notch4 activity in breast CSC compared to more differentiated progenitor cells was increased, while Notch1 activity was reduced. This may indicate that selective suppression of Notch1 and Notch4 may be more effective than non-selective suppression of all Notch receptors, including with γ-secretase inhibitors.
Some food components are able to reduce Notch signaling to a certain extent:
• Retinoic acid lowers Notch signaling through its target genes Hes2, Hey1, Hey2 and Hey5 * *. All-trans retinoic acid (ATRA) has been successfully used in the clinical treatment of acute promyelocytic leukemia by promoting cell differentiation. In breast cancer, ATRA suppresses Notch3 in TNBC cells (MDA-MB-231) in vitro but has no effect on Notch1 in HER2+ cells (SK-BR-3) * *.
• Diallyl trisulfide (DATS) in vitro (60 μM), by inhibiting the expression of Notch ligands Jagged-1 and Jagged-2, suppresses the viability and formation of colonies of ER– (MDA-MB-231) and ER+ (MCF-7) cells without affecting on normal cells (MCF-12A) *. Tumor-grafted mice (MCF-7) treated with DATS showed a reduction in tumor volume compared to control animals without overt toxicity *. Therapeutic plasma concentration of DATS (30 μM) in rats was achieved after an intravenous injection of 10 mg of DATS *.
• Curcumin in vitro (30 µM) interferes with esophageal cancer cell spheroid formation by decreasing Notch1 activation, expression of its Jagged-1 ligand, and its targets Hes-1 *. Pancreatic tumor-grafted mice (MIA PaCa-2) given orally 250 mg/kg of a synthetic analog of curcumin (diflourinated-curcumin) halved tumor size and mass compared to controls by reducing levels of EZH2, CD44, EpCAM, Notch1 and Nanog *.
• Withaferin A, contained in Ashwagandha (Withania somnifera), restores impaired Notch2 function * and prevents carcinogen-induced breast cancer in mouse models (30 mg/day equiv.) *, and inhibits the self-renewal ability of CSC breast tumors in vitro (1 μM) *.
• Genistein in vitro (20 µM) inhibits Notch1 activity in MDA-MB-231 cells *. Supplementation of genistein in rat food (250 mg/kg feed) reduces Notch2 expression in mammary epithelial cells *.
• Oridonin, a diterpenoid isolated from the leaves of (Rabdosia rubescens), in vitro (5 μM) inhibits the proliferation of murine breast cancer (4T1) cells by reducing the expression of Notch1-Notch4 proteins. Intraperitoneal injections of oridonin (5 mg/kg for 21 days) without noticeable toxicity caused a slowdown in the growth of tumor volume (72%) and its mass (84%) in mice *.
• Luteolin, a flavone contained in the root of Celery (Apium graveolens), in vitro (100 μm) shows a high ability to block Notch4 signaling and suppress the proliferation of TNBC; especially populations enriched in CSC *. Apigenin and kaempferol show close, but slightly worse results. The addition of luteolin to paclitaxel increases the latter's cytotoxicity in TNBC. The recommended dosage of luteolin (LutiMax™) is 400-600 mg/day without toxicity.
• Vitamin D and its synthetic analogs in vitro (10-100 nM) inhibit the activity of Notch1, Notch2, Notch3 * * receptors and other components of the Notch signaling axis, including NF-κB, Jag1, Jag2 and HES1 *.
• Resveratrol in vitro (15 µM) reduces Notch1 and Notch2 expression in breast * and ovarian * cells, but it has been reported that it can increase their expression in glioblastoma * and meduloblastoma * cells.
• Psoralidin, a coumarin contained in the seeds of Psoralea (Psoralea corylifolia), in vitro (50 μm) suppresses Notch1 signaling of TNBC cells (MDA-MB-231) *.
Hedgehog are signaling glycoproteins that are critical for embryonic development, tissue and organ patterning, stem cell renewal, cell proliferation and differentiation, and tissue regeneration and repair * *. Hedgehog signaling occurs through two transmembrane proteins, Ptch1 and Smo. The Hedgehog pathway is highly expressed in mammospheres*, indicating their important role in CSC.
• Itraconazole, an antifungal agent *, has been identified as a strong inhibitor of Hedgehog by screening approximately 2'400 molecules previously tested for toxicity or approved by the FDA *. Moreover, itraconazole is able to interact with cyclopamine, that is, both drugs, apparently, act on Smo in different ways. Phase II clinical trial showed that in patients with basal cell carcinoma of the skin, oral itraconazole (2×200 mg for 1 month) reduces, compared with control, cell proliferation by 45%, Hh-pathway activity by 65% and tumor area – by 24% *. The combination of itraconazole and verapamil with 5-fluorouracil in vitro significantly enhanced the therapeutic effect of the latter, mainly due to a decrease in cell viability and proliferation *. Emerging resistance to this therapy can be overcome with PI3K inhibitors *.
• Genistein, soy bioflavonoid, in vitro (30 μM) also reduces the mammosphere and reduces the CSC of the MCF-7 cancer cell line by down-regulating the Hedgehog signaling pathway. Tumor-grafted mice (MCF-7) given intraperitoneal injections of genistein (50 mg/kg daily for 2 weeks) had half the tumor volume and mass *.
• Cyclopamine is a steroidal alkaloid. It targets the Hedgehog pathway, specifically by inhibiting Smo * activation, and inhibits the proliferation of predominantly undifferentiated cells *. In breast cancer, cyclopamine reduces in vitro (300 nM) mammosphere formation and cancer stem cell proliferation *. Cyclopamine in vitro (20 μM) reduces the proliferation of both ER+ (MCF-7) and ER– (MDA-MB-231) breast cancer cells *.
Hippo is a signaling pathway that controls the physical size of organs by controlling apoptosis, cell proliferation, and stem cell function. The positive role of Hippo is to quench the activity of two transcriptional coactivators: YAP and TAZ. Several potential Hippo signaling pathway modulators are listed below, but have not been reported in clinical trials for breast cancer.
• Metformin is a biguanide used for type II diabetes. Metformin directly phosphorylates YAP1 in vitro and in vivo *. The human equivalent dosage is 1'500 mg/day * *.
• Scutellarin significantly inhibited the growth of the grafted ER+ tumor (MCF-7), which was associated with a decrease in YAP expression. Daily intraperitoneal injections of scutellarin (5 mg/kg for 3 weeks) halved the growth of tumor weight and volume in mice, and the combination of scutellarin with 5-fluorouracil reduced this value even more *.
• Apigenin in vitro (30 µM) inhibits migration and stem characteristics of cancer cells by reducing YAP/TAZ activity in TNBC cells (MDA-MB-231 and MDA-MB-436) *. Inoculation of mice with apigenin-treated MDA-MB-231 cells significantly reduced the likelihood of tumor formation, as well as the growth of its volume and mass.
• Ivermectin is an antiparasitic agent that inhibits YAP1 activity in mouse models (10 mg/kg ip for 18 days), but its mechanism of action is unknown * *.
• Chlorpromazine, an older antipsychotic drug, in vitro modulates Hippo signaling and promotes proteasomal degradation of YAP. It is able to directly suppress the stem properties of breast cancer cells, including mammosphere formation, aldehyde dehydrogenase (ALDH1) activity and related gene expression in tumor array cells and in cancer stem cells *.
• Simvastatin is a statin used to lower cholesterol levels. Simvastatin in vitro (1 µM) deactivates YAP/TAZ by expelling them from the nucleus into the cytosol, where they are degraded *.
• Dasatinib, a tyrosine kinase inhibitor that prevents YAP complexation *. The combination of dasatinib with statins in vitro (1+1 µM) mutually enhances YAP/TAZ suppression in cancer cells *.
Nanog is a protein that plays a central role in regulating the differentiation potential and tumorigenicity of cancer stem cells. Nanog expression is regulated by signaling factors Oct4 and Sox2, and its overexpression promotes tumor aggressiveness, invasion, and metastasis.
• Metformin in vitro (1-10 mM) attenuates estradiol-induced Oct4 expression and drastically reduces the size and number of mammospheres, which are grown in a 3D model representing a population of ER+ breast cancer stem cells (MCF-7) *.
• Simvastatin and other statins in vitro (20 µM) inhibit Oct4 gene expression *.
• Vitamin D in vitro (100 nM) reduces Oct4 mRNA and protein levels and inhibits TNBC (SUM159) mammosphere formation *.
• Tretinoin (ATRA) in vitro (20 μM) can suppress the expression of Oct4, Sox2, Nestin and CD44, inhibiting CSC proliferation *.
AHR (Aryl-Hydrocarboxylic Receptor) is a transcription factor that is activated by many carcinogenic factors.
• Omeprazole, a drug used in the treatment of peptic ulcer disease, showed the best in vitro effect * among other studied AHR agonists, including tranilast and sulindac. Of these, only omeprazole reduced the invasion of TNBC (MDA-MB-231) cancer cells in vitro, and significantly inhibited their lung metastases in experimental mice in vivo (100 mg/kg) *.
• Tranilast is a non-toxic anti-inflammatory and anti-allergic agent, AHR antagonist. Tranilast inhibits in vitro (200 μM) the proliferation and formation of colonies and mammospheres of chemotherapy-surviving CSCs in HER2+ and TNBC subtypes *. Treatment of tumor-grafted mice (MDA-MB-231) with tranilast (300 mg/kg for 21 days), compared with control, reduced tumor volume growth by a factor of three and prevented lung metastases. The human equivalent dosage would be 1'800 mg/day, which is 6 times the prescription dose.
NF-κB is a transcription factor that controls the expression of immune response, apoptosis, and cell cycle genes, is important for several key biological processes, including differentiation during embryogenesis, inflammatory and immunological responses, cell proliferation, and survival * * *. In the cytosol of the cell, NF-κB proteins form a complex with the IκB protein, and remain in this inactive state until activated.
• Parthenolide in vitro (5 μm) strongly inhibits the formation of colonies of cancer cells, as well as the mammosphere of the grafted mouse breast tumor (MCF-7) by suppressing the activity of NF-κB *. In mice with grafted ER– tumor (MDA-MB-231), an ethanol solution of parthenolide (40 mg/kg orally), compared with control, increased the 45-day survival rate from 38% to 73%, and in combination with docetaxel – up to 90% *.
• Metformin in vitro (300 µM) selectively targets cancer stem cells through IκB phosphorylation *. The human dosage equivalent to that used in experiments on mice is 200 mg/day.
• Genistein (50 µM) and quercetin (25 µM) in vitro show an antiproliferative effect in cells overexpressing HER2 *.
• Diallyl trisulfide (DATS) also inhibits NF-κB in vitro (10-20 µM); triple negative cancer cell lines (MDA-MB-231 and HS578t) are especially sensitive to it *. DATS is found in garlic at 1-3% by weight; thus, a dietary intake of 10 g of garlic per day (2 cloves) can provide a sufficient concentration of DATS in the body (10 µM) *.
JAK/STAT are transcription factors that provide a cell response to signals from growth factors. Blockade of this pathway results in inhibition of self-healing and expression of lipid metabolic genes *.
• Thymoquinone in combination with paclitaxel, in vitro reduces JAK-STAT signaling. Intraperitoneal injections of thymoquinone (2.4 mg/kg daily) and paclitaxel (1.25 mg/kg weekly) strongly suppressed the growth of mouse TNBC in mice *.
• Metformin in vitro (300 μM) inhibits STAT3 phosphorylation in CSC *.
• Vitamin E (γ-tocotrienol) together with simvastatin in vitro (0.6+5 μM) reduce the proportion of CSC in breast tumors and the expression of the signal mediator STAT3 *.
• Curcumin in combination with EGCG in vitro (10+10 µM) specifically inhibits STAT3 phosphating and STAT3/NF-κB interaction, suppressing the CSC phenotype in the mammary gland *.
PI3K/Akt/mTOR is a signal chain that plays an important role in cell survival, proliferation, migration, differentiation and drug resistance * *. The activation of this pathway in tumors is often disrupted, which allows cancer cells to proliferate and evade apoptosis.
• Garlic extract in vivo interferes with the PI3K/Akt pathway and leads to a significant increase in the cytotoxic effect of chemotherapeutic agents. Intraperitoneal injections of garlic extract reduced mammary tumor growth by 30% in treated mice compared to untreated mice *. The equivalent human dose is 15 ml/day of an extract obtained from about 4 cloves of garlic.
• Quercetin in vitro (60 μm) is an effective inhibitor of PI3K/Akt *. Intraperitoneal injections of quercetin (15 mg/kg 3 times a week for 13 weeks) reduced the growth of grafted TNBC tumor (MDA-MB-231) from metastatic cells by 70% in mice *. The human equivalent dose is 120 mg/day, which is three times the average daily dietary intake of quercetin.
• Sulforaphane in vitro (40 μM) reduces tumor cell clonogenicity and exerts a strong antiproliferative effect via the PI3K/Akt pathway *, with therapeutic serum levels of sulforaphane being achieved with a daily intake of 100 g of raw broccoli.
• Metformin in vitro (300 μm) and in vivo (up to 2 g) activates AMPK and inhibits the enzyme mTOR *.
• Metabolically fast food stimulates mTOR. This is especially true for dairy products, animal proteins, sugar, fruit juices, sugary drinks and other foods with a high glycemic load. Thus, a diet that excludes such foods will certainly be beneficial.
MEK (Mitogen-activated protein Kinase). Although mutations in the Ras/Raf/MEK/ERK signaling pathway genes are relatively rare in breast cancer patients, high activity and higher expression of several gene sequences in this pathway are more common in TNBC than in other breast cancer subtypes * * *. Multiple opportunistic mutations can rapidly develop insensitivity to inhibitors of the PI3K pathway. This requires combination therapy, for example with MEK inhibitors. The dual blockade of PI3K and MEK signals acts synergistically to severely impair the growth of basaloid subtypes of breast cancer in vitro and in vivo *. Some natural substances in vitro are also able to inhibit the Ras/Raf/MEK signaling pathway with some success *, but they are practically unavailable. And the available ones have a very small effect.
• Anthocyanins from black rice in vitro (200 μg/mL) suppress the Ras/Raf/MEK signaling pathway, preventing the metastasis of HER2+ tumors * *. Oral administration of anthocyanides (150 mg/kg) retarded growth and suppressed lung metastasis of TNBC tumor (MDA-MB-453) inoculated into mice *.
• Tanshinon IIA, contained in the root of sage, in vitro reduces the expression of Ras, Raf, VEGFR, HER2, MEK and ERK *. Dosage: 80 mg/day *. Combination tanshinone with piperine may improve the bioavailability of tanshinone.
• D-limonene, a triterpenoid found in citrus peels, reduces Ras and Raf expression levels *. In rat studies, 2.5% peryl alcohol in the diet resulted in regression of 81% of small and 75% of advanced carcinogen-induced mammary carcinomas *. In mice with transplanted mammary tumors, injections of perillyl alcohol (75 mg/kg intraperitoneally every other day for 6 weeks) resulted in both suppression of the growth of the primary tumor and suppression of the spread of metastases to the lymph nodes *. However, phase II clinical trials have not shown any benefit from oral tolerated doses of perillyl alcohol in patients with breast cancer *.
Daily dosage: from 3×1.6 to 4×2 g *, but not higher. Delivery through the nose by inhalation is more comfortable and effective than through the mouth (orally), because swallowing such a large amount of perillyl alcohol causes gastrointestinal disturbances, fatigue and other unwanted side effects.
Twist, Snail are two transcription factors involved in EMT and enhancing metastasis.
• Fisetin in melanoma cells suppresses the expression of Snail1, Zeb1, Twist1 and Slug *. The combination of fisetin (20 µM) with sorafenib (5 µM) is even more effective at reducing cell migration and invasion in vitro. Experiments in melanoma mice (45 mg/kg of fisetin and sorafenib orally) confirmed the results of these studies *.
• Iranian mumiyo (momiai, shilajit, mummy, mumie, mineral pitch) in vitro causes EMT inhibition and suppression of metastasis in breast cancer cells, mainly due to the suppression of TGF-β1 activity, as well as due to the suppression of the activity of Twist1, Notch1 *. The anti-cancer activity of mumiyo was more pronounced for the MDA-MB-231 cell line than for MCF-7, and did not show any effect on normal breast cells.
• Zoledronic acid in vitro(10 µM) reduces Twist and Snail expression in triple negative breast cancer cells *.
• Entinostat in vitro can reverse the epithelial-mesenchymal transition (EMT) of triple-negative breast cancer cells (MDA-MB-231 and HS578t) by reducing Twist and Snail binding to the E-cadherin promoter *.
ALDH1 (Aldehyde Dehydrogenase 1) is a characteristic functional marker of CSC in breast cancer, used for its identification. Positive expression of ALDH1 is approximately 17% in luminal subtype A, 21% in luminal subtype B, 54% in HER2 subtype, 33% in basaloid subtypes, and 18% in non-basaloid subtypes * *.
ALDH plays an important role in stem cell differentiation via retinoic acid *. It enhances the therapeutic resistance of cancer cells as it is able to metabolically inactivate chemotherapy drugs such as cyclophosphamide *.
Finally, NADH produced by ALDH may be an alternative energy source for ATP production in cancer cells * *. Suppression of aldehyde dehydrogenase (ALDH) with gossypol in combination with suppression of mitochondrial complex I with phenformin resulted in depletion of ATP production in cancer cells up to 80%, while normal cells had no loss of ATP production *. This manipulation was accompanied by a regression of the growth of the grafted tumor in animals. Gossypol, however, is not a good choice for humans.
• Disulfiram, aka antabuse, has been used for many years to treat alcoholism – the most powerful inhibitor of ALDH1 known. Its efficacy has been proven in mice at a dosage of 200 mg/kg, equivalent to 15 mg/kg in human terms *. The daily dosage for humans would thus be approximately 1 g/day, which is twice the prescription recommendation for disulfiram and raises concerns about its toxicity even with only 20% oral absorption. In clinical trials, an oral dose of 120 mg/day has been used, but disulfiram monotherapy has not been sufficiently successful in terminal patients *. Antidotes for disulfiram are ascorbic acid and H1 blockers.
• Tretinoin (ATRA) by inhibiting ALDH1 increases the in vitro effect of radiotherapy and chemotherapy (doxorubicin/paclitaxel) on TNBC cells *. In experiments on mice with grafted ovarian tumors, tretionin (0.1 mg/kg once every 2 days for 10 days) reduced the rate of tumor growth by 6 times in treated animals compared to untreated animals *. In human equivalent, this dosage will be 0.6 mg/day.
• Sulforaphane in vitro (1-5 μm) reduces the proportion of ALDH+ cells by 65-80%, and reduces the size (by 8-125 times) and the number (by 45-75%) of primary mammospheres in breast tumors. In TNBC tumors (SUM159) inoculated into mice, sulforaphane (50 mg/kg intraperitoneally for 2 weeks) reduced the number of ALDH+ cells by more than half *. The equivalent human injection dose for these experiments would be 4.2 mg/kg, or 320 mg/day for a 75 kg human.
• Acetylsalicylic acid (aspirin) can reduce the expression of ALDH1, SOX2, OCT4 and NANOG in mouse cancer stem cells (4T1). As a result, the ability to form mammospheres of breast cancer cells is significantly reduced *. In experiments on mice with a grafted tumor, a dosage of 100 mg/kg was used, which in human terms would be 600 mg/day.
IGF-1 (Insulin-Like Growth Factor 1) is a peptide that, in its free (i.e. not associated with glycoproteins) form, stimulates cell division and inhibits apoptosis * *. Meta-reviews of studies show that plasma concentration of IGF-1 is positively associated with the risk of developing breast cancer; at least this applies to estrogen-positive tumors. Women with the highest IGF-1 score had a 28% higher risk of developing breast cancer than women with the lowest score, and this relationship was independent of their menopausal status *. Some research suggests that for breast cancer cells, IGF-1 may be a more powerful growth promoter than estradiol *.
• A vegan diet is able to reduce plasma concentrations of IGF-1 * *. While animal proteins increase IGF-1 levels, most plant proteins decrease it, although the latter depends on the type and amount of protein consumed. A number of studies have found that IGF-1 levels are elevated due to increased consumption of total protein *, sugar, milk protein and calcium *, red meat, total and saturated fats *. Conversely, plant-based and low-protein diets are associated with lower levels of total IGF-1. Retrospective studies show that the mean serum concentration of IGF-1 in vegan women is 13% lower than in meat eaters and vegetarians *.
• Low protein diet. Manipulation studies confirm the relationship between protein intake and IGF-1 levels: a reduction in daily protein intake from an average of 1.67 to 0.95 g per 1 kg of body weight in just 3 weeks led to a decrease in IGF-1 in the serum of healthy people from 194 ng/mL to 152 ng/mL, i.e. by 28% *. At the same time, even long-term calorie restriction does not reduce the concentration of IGF-1 in the serum of healthy people if protein intake remains high.
A rich dietary source of IGF-1 is milk and dairy products, which can double its blood levels *. Drinking a glass of milk daily increases the risk of breast cancer 7 times more than eating an 85-gram serving of boiled meat daily *.
• A low-fat diet combined with moderate exercise reduced serum IGF-1 in the elderly by 20% in just 11 days, and following this course for several years can reduce it by 55% *.
• Exercise combined with a low-fat (10-15% of total calories), high-fiber (30-40 g per 1'000 kcal) diet reduces serum estrogen (by 34-37%), insulin (by 29%) and IGF -1 (by 19%) in postmenopausal women *, reducing the risk of breast cancer and improving survival rates *.
It should, however, be remembered that attempts to inhibit cancer stem cells may interfere to some extent with the function of normal stem cells.
Stimulation of differentiation. As practice shows, a strategy aimed solely at the destruction of cancer cells often fails. Despite initial success in shrinking tumors, cancer stem cells usually continue to renew themselves. An alternative strategy is to stimulate the final cell differentiation, i.e. transferring them to a mature state with the loss of the ability to divide uncontrollably. Increased differentiation of immature cells makes them more susceptible to chemotherapy and less active for metastasis.
To date, very few molecules are known that can effectively enhance differentiation processes. And almost all of them have not been tested in a clinical setting.
• Vitamin A (ATRA as Tretionin) is the strongest and most proven cell differentiation promoter. Dosage and regimen – according to the doctor's prescription (for leukemia, 40 mg is used twice a day for 30-90 days). Although ATRA (all-trans retinoic acid) is widely used to treat several types of cancer, it can cause cytotoxicity and drug resistance if used at high doses in breast cancer *.
Combining ATRA with other natural substances can reduce their therapeutic dose. Thus, curcumin and ascorbic acid increase the in vitro activity of retinoic acid and help to overcome the development of resistance to retinoid in MCF-7 breast cancer cells * *. The combination of ATRA and omega-3 fatty acids in vitro acts synergistically to enhance growth suppression of both estrogen-positive and estrogen-negative tumors *. Other substances of natural origin are much weaker. Unfortunately, cancer cells can very quickly develop insensitivity to this therapy.
• Vitamin D3 regulates cell differentiation * and provides a synergistic effect with retinoic acid *. In experiments in vitro (100 nM), it suppresses Notch signaling, causing the differentiation of triple negative breast cancer cells *, and also reduces the number of cancer stem cells in breast tumor tissue *.
• Calcium provides conditions for the final differentiation of breast cells; when the level of calcium drops below the critical point, the cells do not differentiate * *. This does not mean that calcium should be taken in large doses; this means that you should ensure its adequate level in the body.
• Paracetamol (acetaminophen) is an anti-inflammatory, antipyretic and analgesic drug. In a screening study covering 250 drugs in clinical use, paracetamol was found to induce differentiation of TNBC cells (MDA-MB-231) in vitro by inhibiting the Wnt/β-catenin signaling pathway and downregulating the multidrug resistance membrane pump MRP. Subcutaneous injections of paracetamol (600 mg/kg) inhibited the growth of the grafted MDA-MB-231 tumor in animals no worse than doxorubicin (4 mg/kg), and their combination acted synergistically, delaying the growth of tumor volume by 4 times *. However, the dose of paracetamol used in this study (human equivalent of 4 g/day) is at the limit of the usual clinical dose and may cause hepatotoxicity *.
• Arsenic trioxide (As2O3) in vitro at a low concentration (0.1-1 µM) can significantly promote the differentiation of leukemic cells (HL-60), but at a higher concentration (2.5-5 µM) this ability is lost *. Low concentrations of arsenic trioxide in vitro (0.5 μM) lead to a moderate decrease in viability and increased differentiation of various breast cancer cell lines (MCF-7, MDA-MB-231, T-47D and BT20). At higher concentrations, the proapoptotic effect of arsenic trioxide begins to manifest itself, while the MCF-7 and MDA-MB-231 lines are more sensitive than the T-47D and BT-20 lines.
The concentration of 2 μM arsenic trioxide in breast cancer cells is most effective, and involves three therapeutic mechanisms: growth inhibition, differentiation and apoptosis. The human equivalent dosage, which corresponds to the concentrations of arsenic trioxide in these experiments, is 0.15 mg/kg *. In addition, arsenic trioxide can restore ERα expression in ERα-negative breast cancer cells and render them responsive to endocrine therapy *.
• Butyrate, also known as butyric acid, one of the short chain fatty acids produced by intestinal bacteria, has been shown in vitro to be highly differentiated by cancer cells. However, in clinical studies, systemic administration of butyrate to patients with acute leukemia, even at high doses (500 mg/kg), did not show any effectiveness due to its very rapid metabolism (about 6 minutes) *. Topical administration for colon cancer was more effective.
• Flaxseed enhances mammary differentiation in mice. Feeding mice a diet containing 10% flaxseed or an equivalent level of its major lignan markedly stimulated cell differentiation and healthy mammary gland formation in the animals *.
• Mefloquine, an antimalarial drug, in vitro (10 µM) stimulates stem cell differentiation *.
• Phytic acid (IP6, inositol hexaphosphate), a vitamin found in vegetables and grain husks, in addition to reducing cell proliferation, also promotes in vitro (10 mM) differentiation of malignant cells *. Animals taked IP6 with drinking showed a significant reduction in the incidence and multiplicity of tumors *.
• Lycopene (5 μM)*, quercetin (5 μg/mL) and genistein (15 μg/mL) * increase Cx43 expression in vitro and inhibit breast cancer cell proliferation.
Regulation of metabolic pathways. Development, progression and metastasis of breast cancer most often occur through the activation of various signaling pathways * such as the estrogen receptor, growth factor receptor, cellular metabolic pathways and interactions between them.
Breast cancer is the result of pre-existing congenital or, more commonly, metabolic disorders *. The subsequent restructuring of cancer cell metabolism is a response to the requirements of the developing tumor – from a precancerous state to an advanced cancer. In addition, different types and subtypes of tumor show different metabolism *. Thus, triple-negative breast cancer uses mainly glycolytic energy production, while ER-positive breast cancer uses mitochondrial energy. However, in most subtypes, the processes of glycolysis and glutaminolysis, which are necessary for cell proliferation, are to some extent increased, and which can be a convenient therapeutic target.
The creation of a new cell requires fatty acids to form its cell wall, nucleotides and amino acids to create the cell's proteins, and energy to power the construction process.
• Radicicol, an antibiotic, in vitro (13 μm) binds and inhibits ATP citrate lyase (ATP citrate lyase) *. Another antibiotic, antimycin A, in vitro (30 μM), in addition to inhibiting ATP-citrate lyase, reduces the production of ATP in mitochondria.
• Epigallocatechin-gallate (EGCG) * and rosmarinic acid *, inhibiting the pentose-phosphate pathway of glycolysis, interfere with the production of nucleotides in vitro, and sugiol, contained in the root of sage, does the same in vivo (equiv. 200 mg/day) *.
Bioenhancers (bioavailability enhancers) are substances that by themselves do not have significant pharmacological activity, but when combined with therapeutic substances, they increase their bioavailability and effectiveness.
Certain plant compounds improve the pharmacokinetic parameters of many pharmaceuticals *. They help to overcome the main reasons for the low efficiency of drugs – poor absorption when taken orally, rapid metabolism during the first pass through the liver and their efficient pumping out of the cells. Plant bioenhancers are readily available, safe, reduce drug dosages, and minimize the cost of treatment.
• Piperine, the main alkaloid contained in the seeds of Black Pepper (Piper nigrum) and Long Pepper (Piper longum), is the most studied bioenhancer, which, in addition, has an independent antitumor effect *. Piperine has a dual effect – not only does it help the absorption of drugs in the intestines, but it also prevents P-glycoprotein from removing them from the cells *. In addition, piperine inhibits glucuronidation *. Piperine enhances the bioactivity of several categories of drugs – antibiotics, antiviral, antifungal, anti-inflammatory, cardiovascular and others. In various animal studies, piperine also enhanced the effectiveness of EGCG * and curcumin *. The dose of piperine in these experiments, equivalent to a human, will be about 120 mg/day, but it seems to be at least 500 mg/day *.
• Curcumin, the main curcuminoid from Turmeric (Curcuma longa), can be used as a bioenhancer for antimicrobial and anticancer drugs. In addition, he, and in itself is known for its antitumor properties. Curcumin inhibits hepatic drug metabolism enzymes (CYP3A4) * and impairs P-glycoprotein, which ejects drugs that enter cells. Strengthening the action of the main therapeutic agents is not only ensured during their intake, but only after a few days of preliminary intake of curcumin *. Curcumin increased the bioavailability of docetaxel approximately eightfold in rats *. The human equivalent dose would be approximately 1'200 mg/day * *.
• Quercetin is a flavonoid found in highest amounts in elderberries, cranberries, onions, apples, citrus fruits, vegetables, leaves, and some cereals. Quercetin inhibits cytochrome CYP3A4 and P-glycoprotein * with a dual effect of increasing drug bioavailability. This increases the bioavailability of tamoxifen by 15% compared to the control group *. In other studies, quercetin significantly enhanced the therapeutic effects of doxorubicin *, irinotecan * and etoposide * in animals. The human equivalent doses in these studies were 150-200 mg/day.
• Gingerol (6-gingerol) is a polyphenol found in the root of Ginger (Zingiber officinale), which has some anti-inflammatory * and anti-tumor * activity. Ginger extract enhanced the antitumor effect of doxorubicin in mice with mammary carcinoma *. The dosage of ginger extract as a bioenhancer for humans is 10-30 mg/kg, i.e. approximately 1'500 mg/day *.
• Luteolin and other polyphenols from Cumin (Carum Carvi) seeds act by inhibiting P-glycoprotein *, but also exhibit anticancer effects on their own *. The effective dose of cumin extract is 10-30 mg/kg, and luteolin is 2-20 mg/kg *.
• Carvone (carvone) and limonene are terpenoids contained in the seed of Cumin (Carum Carvi). Cumin seed extract shows its bioenhancing effect at a dosage of 5-100 mg/kg, and its active part at 1-55 mg/kg *.
• Glycyrrhizin is a saponin glycoside extracted from the roots and creeping shoots of Licorice (Glycyrrhiza glabra). Glycyrrhizin promotes the movement of drugs through cell membranes into cells and increases their plasma levels, effectively enhancing the action of antibiotics, anti-infective and anti-tumor agents (such as taxol). The enhancing activity of glycyrrhizin depends on its conversion by the intestinal bacterial enzyme β-glucuronidase to glycyrrhetinic acid.
Unfortunately, there are very few publications on this topic, and there are almost no results of clinical studies that allow making appropriate recommendations. It is impossible to determine the exact dosage of the enhancer in each specific case, because it varies depending on many factors, including the type of drug used.
In addition to chemical bioenhancers, there are also other, as yet unproven methods for enhancing the local absorption of therapeutic agents:
- irradiation of the target area with red light emitted by the LED (~ 620 nm);
- energetic massage of the target area – at least 1-2 minutes 4 times a day;
- applying a negative electric field to the target area, and a positive pole to the remote area – 4 times a day from 2-5 connected 9-volt batteries (total 18-45 volts), if allowed;
- applying the south pole of the magnetic field (800-3'000 gauss) – for 1-2 minutes on the target area of the body 4 times a day;
- acupuncture – 1-2 times a week to the target point or area where there is no infection *.
A small number of studies have been devoted to the effect of the magnetic field on tumors and on the enhancement of primary therapy. A limited number of publications reported a decrease in tumor growth rates in animals under the influence of a pulsating constant magnetic field. However, the theoretical basis of such therapy has not been developed. The reasons for this effect are unclear, side effects are unknown, and there are many versions about the treatment protocol.
Overcoming drug resistance. Therapeutic agents are effective at the beginning of therapy in 90% of cases of primary breast cancer and in 50% of cases of metastasis. However, after a certain period of time, the disease returns in 30% of primary cancers. In this case, resistance to therapy is not only common, but expected. Once metastasis occurs, the possibility of a cure is very limited. The average 5-year survival rate for these patients is 20%, and the average life expectancy varies from 12 to 24 months *. The mechanisms of resistance of cancer cells to each of the drugs may differ *.
It is believed that the main cause of recurrence is the presence of cancer stem cells, which are characterized by resistance to chemotherapy, radiation and other treatments *. There are many genetic and cellular adaptations that render CSCs resistant to commonly used therapies. This is, in particular, a state of relative rest and a slow cell cycle; impaired absorption of chemotherapy drugs; high activity of membrane transporters and aldehyde dehydrogenase; effective DNA repair; expression of anti-apoptotic proteins; resistance to oxidative stress and some others *.
Some substances, acting by various mechanisms, allow counteracting the drug resistance of cells, sharpening their sensitivity to chemotherapeutic agents.
• Metformin reduces the energy capacity of transport pumps (such as P-gp *), pumping toxic substances out of the cell, thereby enhancing the effect of other therapeutic agents. The combination of doxorubicin and metformin kills not only tumor array cancer cells, but also breast cancer stem cells *, and virtually eradicates the tumor in mice.
The combination of metformin with aspirin in vitro (10+5 mM) has a strong synergistic inhibition of tumor growth due to the suppression of the anti-apoptotic proteins Mcl-1 and Bcl-2 and the activation of the pro-apoptotic proteins Bim and Puma. Intraperitoneal injections of metformin (200 mg/kg) with aspirin (60 mg/kg) three times a week for 28 days reduced the growth of the grafted pancreatic tumor in mice by a factor of three compared to control *. In human terms, this would be 1'500 mg metformin and 400 mg aspirin.
• Thymoquinone reduces the ability to repair a damaged DNA molecule and weakens drug resistance. In mice, thymoquinone (100 mg/kg) caused DNA damage in leukemic cells (WEHI-3) by arresting the cell cycle in S-phase *. Patients with metastatic or non-responding breast cancer treated with thymoquinone (1-10 mg/day) reduced tumor markers by 25% from baseline within 2 weeks *. Thymoquinone was well tolerated at doses up to 2'600 mg/day with no toxicity or side effects.
• Sulindac, a non-steroidal anti-inflammatory drug (600 mg), may inhibit the MRP1 membrane pump of multidrug resistance, thereby enhancing the effects of epirubicin in patients with various types of cancer *.
• Aminazine (chlorpromazine) and low concentrations of acepromazine inhibit in vitro (0.9 μM) the activity of heat shock protein HSP27 * * *. Colon tumor-grafted mice treated with injections of chlorpromazine (10 mg/kg every 2 days for 2 weeks) slowed the growth rate of the tumor mass by half *.
• Noscapine is an antitussive opioid that helps overcome resistance to doxorubicin in mice (300 mg/kg) and inhibits the growth of TNBC tumor by 3-4 times *. Noscapine and its analogues block cell division by binding tubulin during mitosis. In rodent studies, noscapine and two other noscapine analogues alone significantly inhibited grafted tumor growth and survival without any detectable toxicity * *. It is very valuable that, unlike standard chemotherapeutic agents, noscapine does not impair the immune surveillance system, as can be judged by the unchanged number of B and T cells among the groups that received noscapine and did not receive it.
• Wogonin reduces the activity of the heat shock protein HSP90 and the level of its client proteins (such as EGFR, CDK4 and Survivin *). Experiments with mice have shown the activity of wogonin in suppressing the angiogenesis of the grafted mammary tumor (equiv. 10 mg/kg) *.
• Simvastatin and other statins in vitro (20 µM) activate the pro-apoptotic Bax genes and downregulate the anti-apoptotic Bcl-2 and Survivin genes in abnormal and cancerous breast cells *.
• Silibinin (200-400 μM), CAPE (25 μM) and vitamin C (1 mM) * in vitro reduce intracellular energy production by blocking aerobic respiration, thereby significantly inhibiting the formation of the mammosphere of ER+ tumors.
• Salinomycin, an ionophore antibiotic, strongly inhibits the membrane pump ABCB1 (P-glycoprotein) *. It also reduces in vitro (1 μM) c-FLIP expression and induces apoptosis via activation of the death receptor DR5 *.
• Piperine, capsaicin *, resveratrol *, EGCG * * and other polyphenols * * * * in vitro also inhibit the transport activity of P-glycoprotein, but incomparably weaker. Piperine is also a strong inhibitor of MRP1 *.
• Myricetin in vitro (20 μm) * also inhibits the work of the multidrug resistance proteins MRP1/MRP2.
• Curcumin *, berberine *, EGCG * as well as flavonoids acting as natural AHR ligands (kaempferol, chrysin, genistein, naringenin) can be used to inhibit the BCRP (breast cancer resistance protein) membrane pump * * *.
• Giloy (Tinospora cordifolia). Tinospora dichloromethane extract significantly inhibits ABC transporters, very effective against drug resistant various epithelial tumors *.
• Antabuse (disulfiram) and other ALDH inhibitors in vitro increase the sensitivity of cells to chemotherapy drugs, in particular cyclophosphamide * and gemcitabine *.
• Celecoxib in vitro counteracts the activation of the Akt/Survivin pathway, thereby inhibiting apoptosis and autophagy *.
• Epigallocatechin gallate (EGCG) in vitro (50 µM) is a natural inhibitor of the heat shock protein HSP90 *.
Mammary CSCs, regardless of their clinical subtypes and tumor heterogeneity, are extremely sensitive in vitro to abolish repression of the TRAIL proapoptotic pathway *.
• Magnolol suppresses the NF signaling pathway and activates pro-apoptotic signals including DR5, Bax and caspase-3 *. The combination of magnolol with another magnolia polyphenol, honokiol (20+20 mg/kg), acts synergistically and several times reduces the development of grafted glioma in mice *.
• Butyrate increases the expression of the cell death receptor DR5 *. As an exogenous additive, sodium butyrate exhibits poor stability; however, the level of endogenous butyrate can be raised by normalizing the intestinal microflora by feeding butyrate-producing bacteria with plant fiber.
Overcoming intratumoral pressure. Tumor-associated inflammation causes increased capillary leakage, and increased lymphatic fluid increases local hydrostatic pressure within the tumor. In addition, tumor angiogenesis forms defective blood vessels, which are also characterized by leakage, and even more defective lymphatic vessels, which do not cope well with their functions. Increased permeability of the vascular wall, together with a decrease in lymphatic function, leads to an increase in intercellular pressure within the tumor.
Increased pressure in the tumor, as well as its increased rigidity, creates a microenvironment that promotes cell proliferation * and increases the invasiveness of tumor cells *.
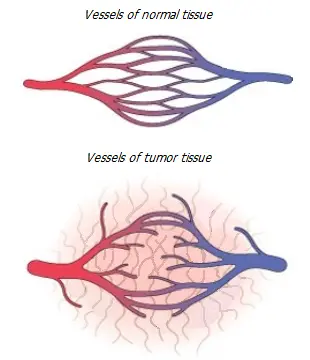
In addition, fibrosis, poor fluid circulation and increased intratumoral pressure prevent the penetration of therapeutic agents into the tumor in a natural way, and also tend to squeeze them out. This is one of the most important reasons why the treatment of the tumor by the administration of various chemicals fails. We can create the most effective drugs in the test tube, but the problem of getting them to the intended target can completely deprive them of the ability to fight the tumor.
To overcome this problem, it is possible to inject therapeutic drugs directly into the tumor. Local targeted delivery can significantly reduce the side effects of therapy and enhance its effectiveness. Invasive methods may include injections and/or catheters, as well as intratumoral capsules that slowly release therapeutic substances. Unfortunately, traumatic drug delivery methods violate the integrity of the basement membrane, which inhibits the spread of tumor cells.
More preferred are non-invasive methods of administering therapeutic agents, which are based on overcoming hydrostatic pressure using pressure of a different nature. These can be, for example, sonophoresis – an increase in tissue permeability under the action of acoustic energy of ultrasound, or iontophoresis * – delivery of drugs to the tumor not along a concentration or pressure gradient, but under the influence of direct electric current energy. Electrophoresis is an old proven technology that has been successfully practiced in other areas of medicine, but in recent years has also been used in experiments against malignant tumors * *.
Local administration of active agents allows them to be delivered to the target without first passing through the liver, as well as to increase their concentration without increasing the load on other organs and tissues, thereby reducing negative side effects. However, both of these promising methods have not yet been introduced into widespread oncological practice. One of the obstacles to this is the fact that transcutaneous electrophoresis allows to provide therapeutic concentrations at a distance of several millimeters from the electrode. This could be of benefit in malignant skin lesions, but reaching breast cells with therapeutic agents in classical electrophoresis seems doubtful. The solution to this problem is seen in the injection of active substances not into the tumor itself, but in the immediate vicinity of it, and then the effect of iontophoresis to force them to penetrate into the tumor. But it still remains in the field of bold proposals.
Iontophoresis, however, can be more effective if the active substances are forced to move not directly through the skin and tissue, but through the vessels, in particular, along the milk ducts. This approach may be useful in Paget's disease. For deep delivery into the mammary gland, therapeutic agents will need to be previously injected into the ducts through the nipple.
Transductal electrophoresis might be useful in the following situations:
• Preoperative treatment of the mammary gland – by saturating the tissue with precisely measured doses of progesterone, doxycillin or other substances that can improve the outcome of subsequent surgical intervention.
• Postoperative treatment – by introducing anti-inflammatory and reparative substances, antioxidants and vitamins, as well as clarithromycin, diclofenac, iodine, vitamin D, retinoic acid.
• During general chemotherapy, in non-advanced primary breast tumors, topical administration of vasodilators and stromal modifiers, including proteolytic enzymes, could improve target tissue saturation with the primary therapeutic agent.
For the destruction of cancer stem cells – salinomycin or niclosamide, metformin, itracosanol, disulfiram, diclofenac, vitamin D, retinoic acid.
• During radiation therapy, local saturation of the tissue with oxygen, iron and copper ions, and other oxidizing agents could presumably increase the radiation-induced oxidative stress, and thus make it possible to reduce the therapeutic power of ionizing radiation.
In addition to radiation therapy – paracetamol, metformin, diclofenac, dibazol.
For tissue repair after radiation therapy – resveratrol, curcumin.
• In the inter-therapeutic period, electrophoresis can regulate the extracellular acidity of a glycolytic tumor by delivering alkaline ions, including rubidium and cesium, into and in close proximity to it.
To destroy the remaining cancer cells of the tumor array – viscumin, baicalein, tanshinon, parthenolide.
As inhibitors of glycolysis – wogonin, clotrimazole.
As OP inhibitors – doxycycline, azithromycin, metformin.
As anti-inflammatory agents – curcumin, acetylsalicylic and boswellic acids.
• In all cases, as enhancers – dimethyl sulfoxide, dexamethasone, dibazol.
Despite all its promise, such therapy is still at the stage of theoretical development.
Nanocarriers. An ideal drug delivery system should ensure drug stability, targeting to a specific site in the body, and controlled release after delivery to the end point.
Once introduced into the body, therapeutic agents must overcome a number of obstacles before they can achieve their goal. They need to remain intact, avoid binding and removal from the body, evade absorption by immune cells, accumulate in their target in sufficient quantities, and only after that they can manifest their therapeutic effect.
And taken orally, they must first penetrate the intestinal wall into the bloodstream, which presents certain difficulties for hydrophobic substances. Not surprisingly, many effective in vitro substances turn out to be completely unimpressive in clinical studies.
In recent years, the interest of researchers has turned to the creation of nanocarriers as a promising mechanism for the transport of drugs. The effectiveness of nanocarriers as drug delivery systems is strongly related to their structure and characteristics.
Requirements for nanoparticles.
The size of nanocarriers is important for their movement through the bloodstream and delivery to the tumor tissue *. The optimal range of nanoparticle sizes is 30–200 nm. Nanoparticles with a size of 100-150 nm have 4-6 times higher absorption by the tumor than particles with a diameter of less than 30 or more than 300 nm *.
It has been observed that particles below 20-30 nm can be rapidly excreted through the kidneys; particles larger than 50 nm do not cross the blood/brain barrier well; particles larger than 100 nm tend to accumulate near the walls of the vessel; particles larger than 200 nm weakly penetrate the tumor endothelium; particles larger than 3 microns are subject to enhanced phagocytosis; and particles larger than 4 microns can be retained in the smallest capillaries of the vasculature * *. In the range of 120-200 nm, nanoparticles are not perceived by cells of the reticuloid endothelial system and, due to this, they avoid rapid filtration by the liver and spleen *.
The Ζ-potential is determined by the charge on the surface of particles suspended in a colloidal solution, which is blood. The more negative the value of this indicator, the stronger the particles repel each other, the less their tendency to agglomerate and the higher their stability. On the other hand, particles with a small positive charge are absorbed by cells faster. The value of the ζ-potential of the nanocarrier from -30 mV to +5 mV is considered acceptable.
Biocompatibility and biodegradability of materials are also important for the effectiveness of therapy.
Classes of nanocarriers. The most common materials are proteins, cyclodextrins, liposomes, polymers, polymer-lipid hybrids, dendrimers, hydrogels, phase change materials, and inorganic materials. Each of them has its pros and cons. Consider the most easily implemented of them.
Cyclodextrins are about 70 nm in size carbohydrates with a conical molecular structure that has a lipophilic interior and a hydrophilic exterior. This design makes it possible to harbor hydrophobic substances in the interior of the cyclodextrins, improving their bioavailability. Loading of cyclodextrins is extremely easy, and their capacitance decreases in the following order: 2-hydroxypropyl-β-cyclodextrin → methyl-β-cyclodextrin → β-cyclodextrin → γ-cyclodextrin *. For all their advantages, cyclodextrins are not closed containers, and can lose their contents just as quickly.
Liposomes are spheres formed by a double lipid layer similar to a cell membrane. Liposomal structures are easily produced and allow the transport of both hydrophilic substances (in the inner compartment) and lipidophilic substances (inside the layer). However, liposome technology produces relatively large particles (over 200 nm).
Solid lipid nanocarriers are spheres with a single-layer lipid layer surrounding therapeutic substances. This technology, compared to liposomes, usually produces smaller particles (50-200 nm) with a higher concentration of transported substances. It is well suited not only for injection, but also for oral administration, however, it is capable of loading only lipidophilic molecules.
Although the lipids themselves are biocompatible and non-toxic, the technology for preparing lipid shells often requires organic solvents. Homogenization can alternatively be used, but it requires high energy processes such as high pressure extrusion, prolonged (up to 3 hours) high shear mixing, or prolonged sonication of the liquid mixture.
Since nanoparticles exhibit high permeability, including penetration through the blood/brain barrier, special requirements are placed on the purity of the raw materials and technologies used.
Modifications of nanocarriers. The release of the contents of lipid nanoparticles occurs by erosion of the lipid membrane and diffusion of their contents. Without additional improvements, lipid structures show instability, and in addition, they are quickly detected by macrophages. Coating liposomes with polyethylene glycol (5-10%), or similar substances, increases their stability, and at first masks liposomes from recognition by immune cells.
The targeting of nanocarriers can be enhanced by attaching to their surface ligands with high specificity for receptors and other targets that are specifically overexpressed on the surface of breast cancer cells. These can be, for example, estriol, hyaluronic acid, folates, retinoids.
The folate receptor is overexpressed on TNBC cells, so folate-containing liposomes will have greater uptake by cancer cells *. And hyaluronic acid simultaneously functions both as a hydrophilic stabilizer and as a molecule showing high affinity for the CD44 receptor (transmembrane glycoprotein type 1), the expression of which is increased in cancer cells * *.
The use in the shell of nanocarriers of materials that lose their stability during hypoxia and/or increased acidity, observed in tumor tissue, can additionally increase the effectiveness of therapeutic agents that have achieved their goal. However, tumor cells are not the only cells in the body that divide under conditions of high acidity. Therefore, it would be more profitable to use materials that respond to controlled external influences, such as light, ultrasound, temperature, electromagnetic field, etc.
Increased vascular wall permeability in the tumor, together with poor lymphatic drainage, may facilitate passive seepage into the tumor and subsequent retention of nanoparticle-encapsulated therapeutics. However, delivery of agents to nascent small tumors that do not have disturbances in growing vessels is unlikely to benefit from the vascular «leakage» effect.
Nanocarriers are already being used in clinical settings, increasing the efficacy of many chemotherapeutic agents * and reducing their side effects. Due to their success, further growth in the use of nanocarriers is expected.
An example of the preparation of nanoparticles loaded with curcumin based on cyclodextrin.
Beta-cyclodextrin (40 mg) is dissolved in 8 ml of deionized water in a 20 ml glass container, adding 40 ml of a 30% curcumin solution in an organic solvent (preferably ethanol). The resulting mixture was stirred overnight at 400 rpm without a lid to evaporate the solvent. Store in the dark at 4°C for several days *.
These nanoparticles increase the solubility of curcumin by 180 times *, however, they do not prevent curcumin from being rapidly metabolized.
An example of the preparation of lipid nanoparticles loaded with curcumin by a high shear hot homogenization method.
Curcumin is taken ready-made, or extracted from turmeric powder using a Soxhlet extractor using dichloromethane as a solvent (or, as solubility deteriorates, acetone → ethyl acetate → methanol → chloroform → ethanol → dichloroethane → isopropanol → ether → benzene → hexane). Of this list, ethanol seems to be the most acceptable. The yield of dry finished product after removal of the solvent should be 2-5% by weight of the feedstock.
Nanoliposomes are produced without the use of solvents, by homogenizing two pre-prepared parts – lipid and water, in a weight ratio of 0.1-30%. In this case, the water part includes 0.5-5% stabilizing surfactants.
To prepare the lipid part, glyceryl monooleate (500 mg) can be taken as a solid lipid, and olive oil (25 mg) can also be used as a liquid lipid. The lipids are heated in a water bath to 80 °С in a glass dish, after which 5 mg of curcumin and 80 mg of lecithin as an auxiliary emulsifier are added to them, mixing thoroughly.
To prepare the aqueous part, 200 mg of polysorbate 80 (Tween 80) per 50 ml of double-distilled water is taken as the main emulsifier, and this mixture is heated to 75°C. The hot water part is added little by little to the lipid part, breaking it up using a homogenizer at 12'000 rpm for 15 minutes at a temperature of 75-80°C. The resulting dispersion is rapidly cooled in an ice bath and transferred to a freezer (-20 °C) for complete cooling. Store at a temperature of +4°C in a dark place for no more than a month. Dosage: 30 g/day, i.e. about 2 tablespoons/day.
The average size of the resulting nanoparticles should be 110 nm at a ζ potential of -44 mV; encapsulation efficiency – 75%; and the volume of curcumin loading into the liposome is about 1%. The IC50 value against ER-positive cancer cells (MCF-7) for nanoparticles can increase more than 20 times compared to pure curcumin *. Irradiation of cells with blue light (420-430 nm) further increases this gap.
Alternatively, fish oil, eucalyptus oil, or sandalwood oil can be used instead of olive oil for the lipid part. The lipid portion can be supplemented with fat-soluble therapeutic agents such as retinol. The water part can be supplemented with other water-soluble substances such as hyaluronic acid or folate (200 μg).
If the speed of the homogenizer is reduced to 5'000 rpm, the procedure will need to be extended to 1.5 hours. Instead of homogenization, ultrasonication can be used for 1-2 hours with continuous stirring, frequency 40 kHz and intensity 15 W/cm2. However, all deviations from the technology described above can significantly change the characteristics and efficiency of nanoparticles.
An example of preparation of lipid nanoparticles loaded with curcumin by hot homogenization and ultrasound * * *.
To prepare the lipid part, 120 mg of cetyl palmitate (vegetable spermaceti) and 180 mg of oleic acid are mixed and heated to a temperature 5°C above the melting point of cetyl palmitate. Next, 15 mg of pure curcumin is dissolved in the hot melt with constant stirring (optionally, plus 15 mg of kalinji oil). To prepare the aqueous part, dissolve 200 mg of polysorbate (Tween 80) in 10 ml of double-distilled water and heat to the temperature of the lipid part (optionally plus 100 µg of folate).
Then a hot aqueous solution, with constant stirring and maintaining the initial temperature, is added dropwise to the lipid part, and homogenized for 3 minutes at a rotation speed of 11'000 rpm, obtaining large lipid particles (about 3 μm). For further crushing of particles, the mixture is treated with ultrasound with a specific power of 100 W/L for 20 min. Particle size reduction occurs under the action of turbulence and cavitation. After that, while continuing to stir, the solution is transferred to an ice bath and quickly cooled to room temperature.
The average size of the resulting nanoparticles should be 120-200 nm at a ζ potential of about -30 mV; encapsulation efficiency is over 80% at 5% curcumin loading.
An example of the preparation of lipid nanoparticles loaded with curcumin using water-miscible organic solvents.
To prepare the lipid part, 150 mg of curcumin, 200 mg of stearic acid and 100 mg of lecithin are dissolved in 10 ml of chloroform or ethanol using ultrasound. To prepare the aqueous part, 200 mg of polyethylene glycol monostearate is dissolved in 30 ml of double-distilled water. Both parts are heated to 75°C.
The molten lipid portion is then added through a G23 injection needle (diameter 0.6 mm) to the hot aqueous portion, stirring at 1'000 rpm. In this case, the organic solvent migrates into the aqueous part, causing the precipitation of nanoparticles. Stirring is continued for approximately 1 hour at 75°C. During this treatment, the organic solvent is completely evaporated and the volume of the mixture is concentrated to approximately 5 ml.
After that, the resulting composition is immediately placed in an ice bath (0-2°C), and 5 ml of cold water are added, stirring at a speed of 1'200 rpm for 2 hours * *.
The average particle size should be 40 nm at a ζ potential of -50 mV. The drug loading and encapsulation efficiency is 23% and 72% respectively. IC50 is 1.5 times higher than pure curcumin. Dosage: 5 g/day, i.e. about 1 tsp/day.
In general, the finished composition should contain approximately:
• 80% double distilled water;
• 10% solid lipids (cetyl palmitate, beeswax);
• 4% liquid lipids (oleic acid, olive oil, lard, coconut oil, linseed oil, kalinji oil, sandalwood oil, fish oil);
• 5% emulsifiers (polysorbate-80, soy lecithin, egg lecithin);
• 1% active ingredient (curcumin, quercetin, resveratrol, retinol, etc.).
However, the characteristics of the nanoparticles will vary greatly depending on the components used, and may not meet the required conditions.
Concluding the review of the means and methods for the destruction of cancer cells, we note that no matter how outstanding the successes in this matter may be, getting rid of cancer cells alone does not mean getting rid of the disease. Cancer cells are only a manifestation of the problem, but not its essence *.